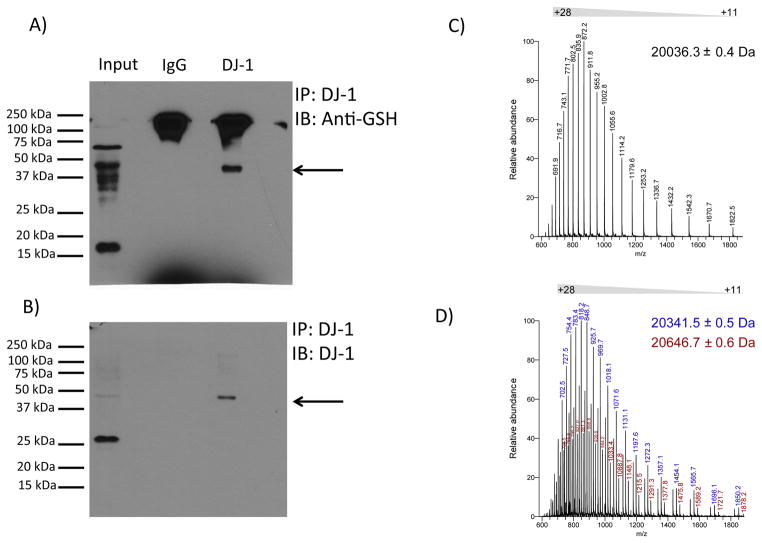
Figure 3. DJ-1 is glutathionylated in vivo and in vitro.
(A) Representative western blot analysis of immunopreciptated DJ-1 from mouse brain homogenate probed with anti-glutathione (GSH) antibody. Replicate analyses of four independent biological samples gave similar results. Arrow denotes a band of immunoreactivity at approximately 45 kDa. (B) Immunopreciptated DJ-1 recognized by anti-DJ-1 antibody. The blot shown in (A) was stripped and re-probed with anti-DJ-1 antibody. Again, this was replicated with the four different samples. Arrow denotes a band of immunoreactivity at approximately 45 kDa. Overlay of the films shown in panels A and B documented coincidence of the 45 kDa band, representing glutathionylated DJ-1. (C) Electrospray ionization mass spectrum of intact recombinant DJ-1. The mass spectrum shows a distribution of charged states (+11 to +28) for intact DJ-1 protein. Deconvolution of the spectrum reveals a protein mass of 20036.3 Da, which is identical to the theoretical mass of recombinant DJ-1. (D) Electrospray ionization mass spectrum of glutathionylated DJ-1. Incubation of DJ-1 protein with 5 mM GSH and 1 mM diamide led to an increase in protein mass of 305-Da (blue ion series, deconvoluted mass = 20341.5 Da) or 610-Da (red ions series, deconvoluted mass = 20646.7 Da), corresponding to covalent modification of DJ-1 with one or two glutathione moieties, respectively.