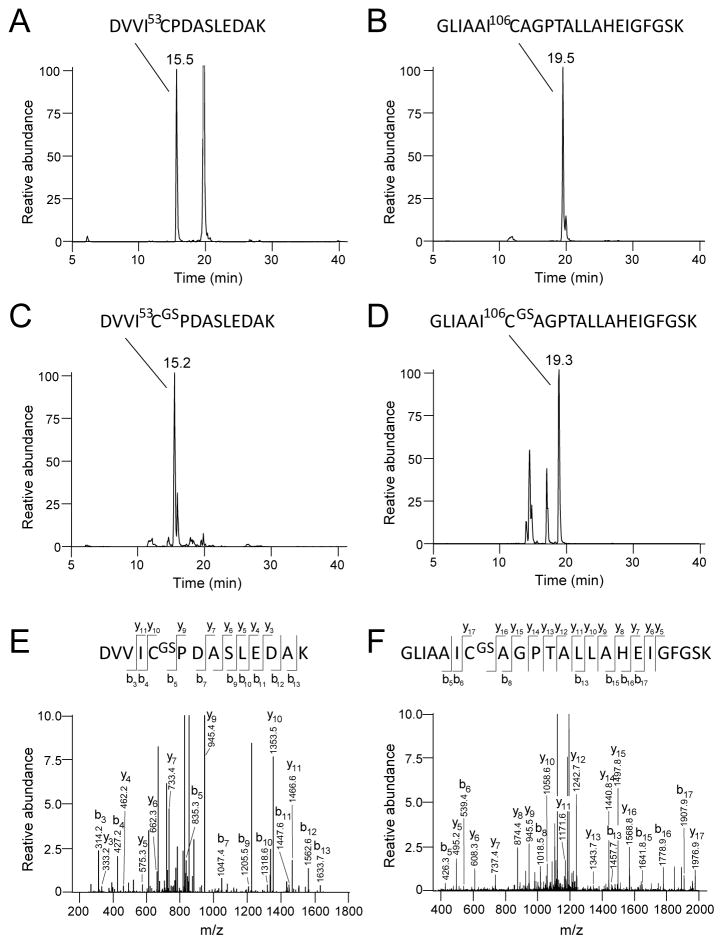
Figure 4.
Identification of the glutathionylation sites of DJ-1 by tandem mass spectrometry. Selective Ion chromatographs of tryptic digest of DJ-1 treated with 5 mM GSH and 1 mM diamide revealed two Cys containing peptides (C) DVVIC53PDASLEDAK (m/z = 766.5 [M+2H]+2) and (D) GLIAAIC106AGPTALLAHEIGFGSK (m/z = 1258.2 [M+2H]+2), with corresponding masses increased by 305-Da as compared to the untreated sample shown in (A) and (B). (E) and (F): MS/MS fragmentation pattern of these peptides identifies Cys53 and Cys106 residues as the sites of glutathionylation. According to Biemann nomenclature, the series of b and y ions are identified in the mass fragmentograms and in the diagrams of the Cys-containing peptides at the top of panels E and F. For example, the site of glutathionylation at Cys-53 is identified by the loss of 408 mass units for the y9 ion versus the y10 ion, corresponding to the loss of the cysteinylglutathione moiety.