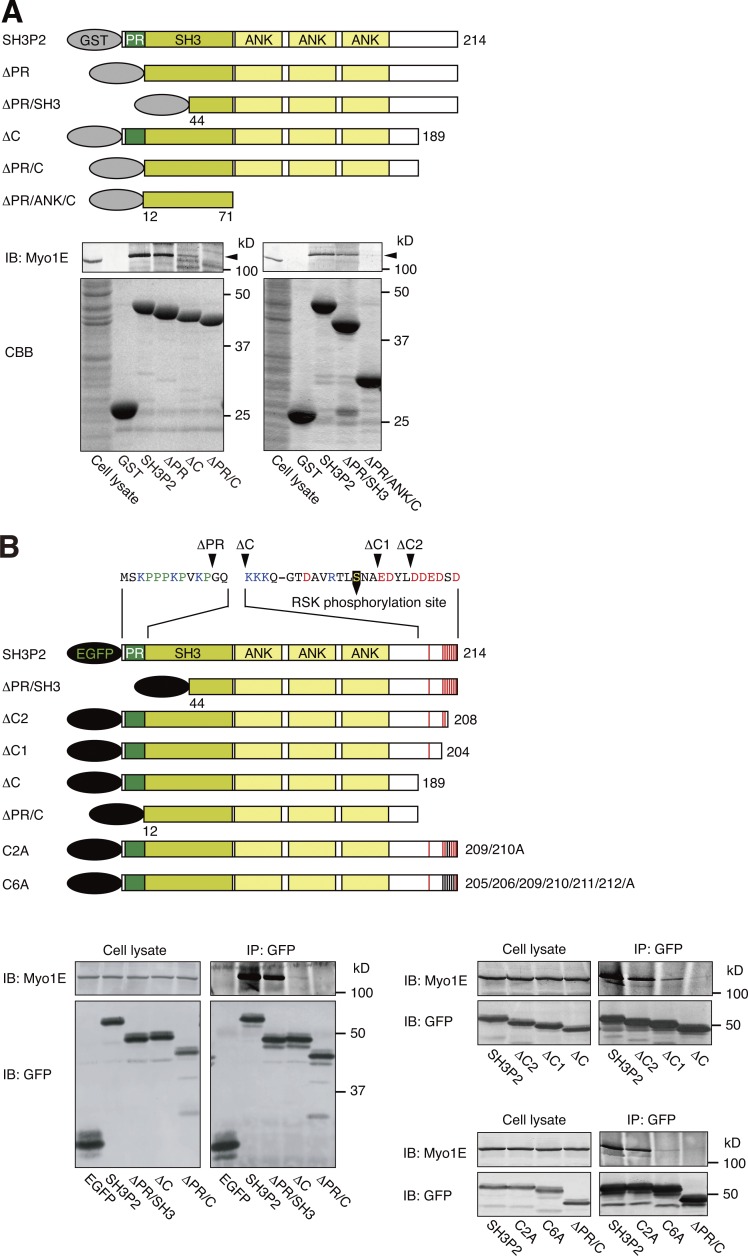
Figure 2.
SH3P2 interacts with Myo1E via its N-terminal proline-rich region and C-terminal acidic amino acid cluster. (A) Glutathione-Sepharose beads coupled with GST or GST-tagged wild-type or indicated mutant forms of human SH3P2 were incubated with MKN1 cell lysates, after which bead-bound proteins as well as the whole-cell lysates (10% of the input for the pull-down assays) were subjected to SDS-PAGE followed by staining with Coomassie brilliant blue (CBB) or immunoblot analysis (IB) with antibodies to Myo1E. Arrowheads indicate the Myo1E band. (B) MKN1 cells were transfected for 24 h with vectors encoding EGFP-tagged wild-type or mutant forms of human SH3P2, lysed, and subjected to immunoprecipitation (IP) with antibodies to GFP. The resulting precipitates as well as the whole-cell lysates (10% of the input for immunoprecipitation) were subjected to immunoblot analysis with antibodies to Myo1E and to GFP. Amino acid sequences of the PR region and the C-terminal acidic amino acid cluster are shown. The RSK phosphorylation site (Ser202), acidic amino acids, basic amino acids, and proline are indicated in yellow, red, blue, and green, respectively. All data are representative of at least three separate experiments.