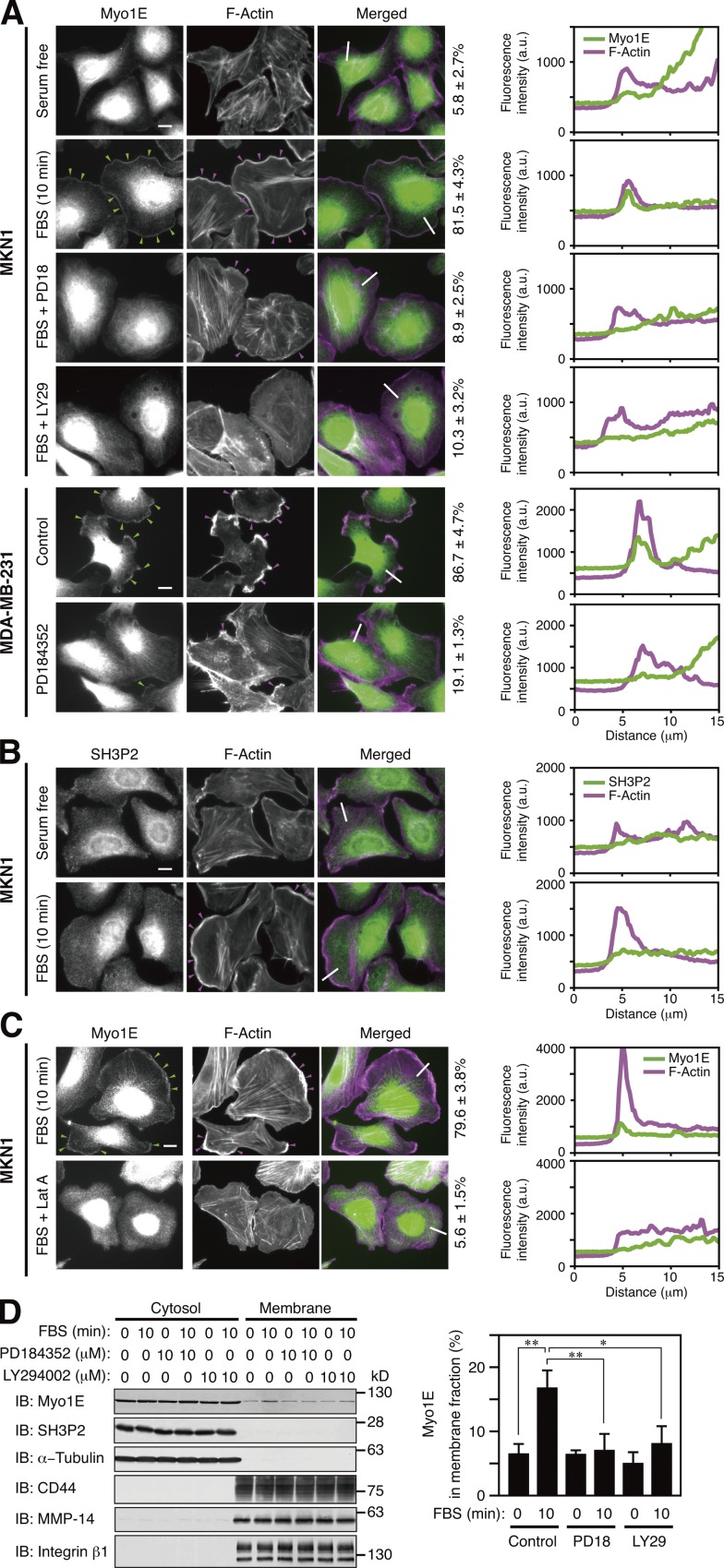
Figure 5.
Myo1E localizes to lamellipodial tips after dissociation from SH3P2. (A–C) MKN1 cells deprived of serum for 12 h were incubated with or without 10 µM PD184352 (PD18), 10 µM LY294002 (LY29), or 50 nM latrunculin A (Lat A) for 30 min and then stimulated with 10% FBS for 10 min. Exponentially growing MDA-MB-231 cells were incubated with or without 10 µM PD184352 for 4 h. All cells were then fixed and stained with antibodies to Myo1E (A and C, green) or to SH3P2 (B, green) as well as with phalloidin (A–C, magenta). Green arrowheads indicate Myo1E colocalized with F-actin, whereas magenta arrowheads indicate lamellipodia. Bars, 10 µm. The percentages of cells in which Myo1E and F-actin were colocalized are shown at the right of the merged images as means ± SD for three separate experiments, with n ≥ 80 (A) or 40 (C) cells in each experiment. Fluorescence intensity profiles along the white lines in the merged images are also shown on the right (a.u., arbitrary units). Data are representative of at least three separate experiments. (D) MKN1 cells deprived of serum for 12 h were incubated with or without PD184352 or LY294002 for 30 min and then stimulated with 10% FBS for 10 min. The cells were then subjected to subcellular fractionation, and 20% of the cytosolic fraction and 50% of the membrane fraction were subjected to immunoblot analysis (IB) with antibodies to the indicated proteins (left). α-Tubulin was analyzed as a cytosolic marker and CD44, MMP-14, and integrin β1 as membrane markers. The proportion of Myo1E in the membrane fraction was determined by measurement of immunoblot signals as mean ± SD values for three separate experiments (right). *, P < 0.05; **, P < 0.01.