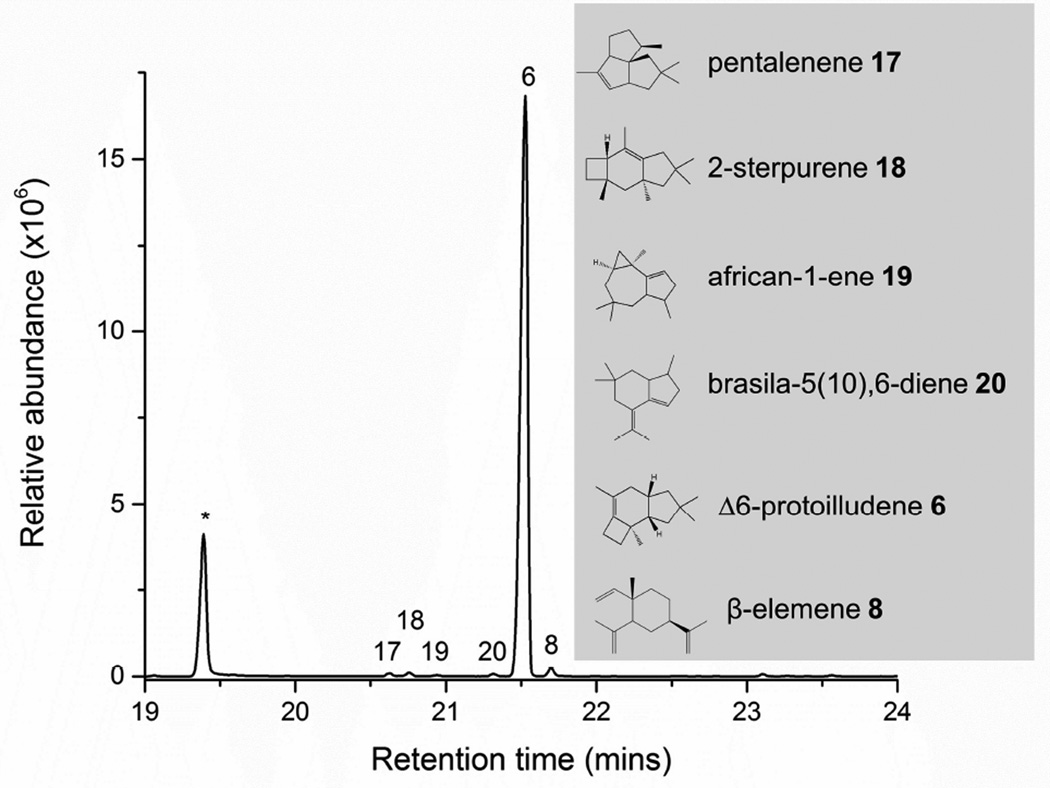
Fig 6. GC/MS analysis of the volatile sesquiterpenes produced by E. coli overexpressing Dia1.
Headspace analysis of E. coli cells overexpressing Dia1 reveals Δ6-protoilludene 6 as a major peak on the chromatogram, as well as minor peaks corresponding to pentalenene 17, 2-sterpurene 18, african-1-ene 19 and brasila-5(10),6-diene 20, which are all derived from a 1,11 cyclization of (2E,6E)-FPP. β elemene 8 is a heat induced rearrangment product of a 1,10 cyclization of (2E,6E)-FPP. Peaks that could be assigned based on comparison of mass spectra to known libraries of terpenes are labelled with numbers, and chemical structures are shown in the grey box. Indole, highlighted with an asterisk (*), is a breakdown product of tryptophan naturally produced by E. coli, and serves as an internal standard.