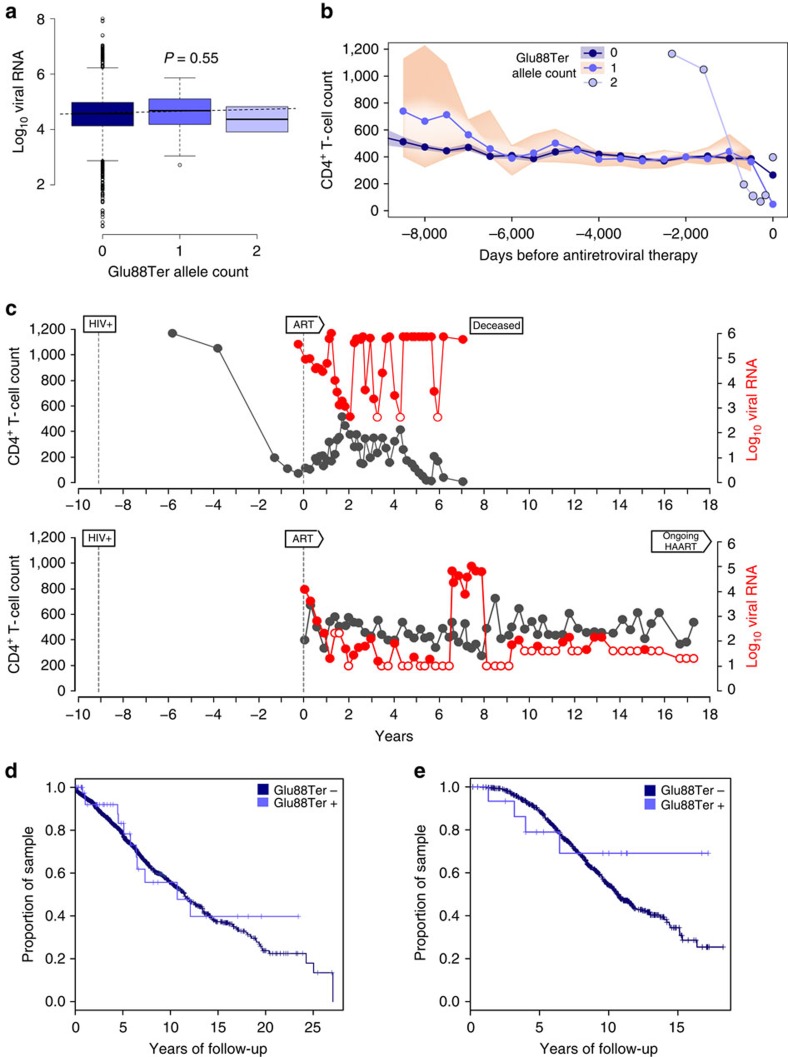
Figure 3. Analysis of association between Siglec-1 Glu88Ter and HIV-1 clinical outcomes in the absence of antiretroviral treatment.
(a) Set point viral load of individuals from the SHCS with or without the Glu88Ter allele (n=2,243). (b) CD4+ T-cell count dynamics of individuals from the SHCS with or without the Glu88Ter allele (n=3,385). CD4+ T-cell counts (cells mm−3) were binned using 500 days windows, counting backwards from the date of antiretroviral treatment start or loss of follow-up. Median CD4+ T-cell values (lines) and interquartile ranges in each bin (shaded areas) are shown for individuals carrying no copies (n=3,305) or one copy (n=78) of the Siglec-1 Glu88Ter allele. Actual CD4+ T-cell values are shown for the two Siglec-1 Glu88Ter homozygotes. (c) Plasma viral RNA level (cp ml−1) and CD4+ T-cell count (cells mm−3) dynamics of the two Siglec-1 Glu88Ter homozygotes. The dates of first HIV-1 positive report and of ART initiation are depicted. Open circles indicate negative values bellow the represented detection level. (d) Time to AIDS measured in the SHCS cohort (n=2,458 common allele Glu88; n=53 Glu88Ter including 52 heterozygous and 1 homozygous individuals) or (e) the MACS cohort (n=401 common allele Glu88; n=12 Glu88Ter which were all heterozygous).