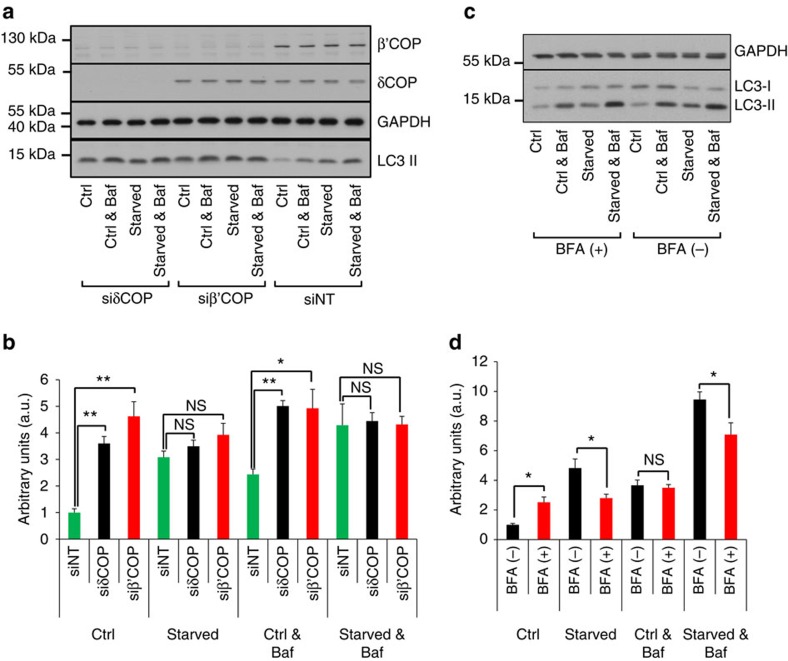
Figure 2. COPI complex promotes lipidation of LC3.
(a) HEK293 cells were transfected with siRNA targeting δCOP (siδCOP), β'COP (siβ'COP) or non-targeted (siNT), starved in the presence or absence of 0.1 μM bafilomycin and lysed. Lysates were assessed by western blotting using antibodies against LC3, β'COP, δCOP and GAPDH. Note that siRNA depletion of δCOP decreases the stability of β'COP. (b) Western blots from a were quantitated and values of LC3 II normalized to GAPDH and then to the siNT at control conditions are shown as means±s.e.m. From five independent experiments. One arbitrary unit (a.u.) equals the average of LC3 II in fed cells. (c) HEK293 cells were pre-treated for 3 h with 3 μg ml−1 BFA, starved for 1 h in the presence or absence of BFA and bafilomycin and lysed. Lysates were assessed by western blotting using antibodies against LC3 and GAPDH. (d) Western blots from c were quantitated and values of LC3 II normalized to GAPDH and then to the control conditions in the absence of BFA are shown as means±s.e.m. From six independent experiments. One arbitrary unit (a.u.) equals the average of LC3 II in fed cells. Significance levels were determined with repeated-measures analysis of variance followed by Holm–Sidak's multiple comparison tests. NS non significant; *P=0.05%; **P=0.01%.