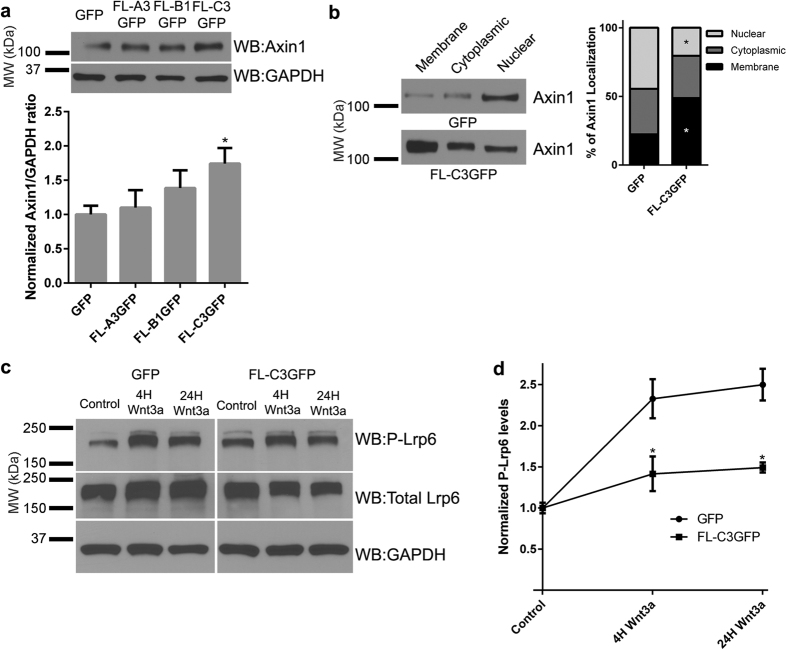
Figure 7. Interaction between γ-Pcdh C3 and Axin1 stabilizes Axin1 at the membrane and prevents phosphorylation of Lrp6.
(a) Representative Western blot showing increased endogenous Axin1 levels in HEK293 cells overexpressing FL-C3GFP in comparison to GFP, FL-A3GFP, or FL-B1GFP. The band intensity of Axin1 was quantified and normalized to that of GAPDH in the same lane. Means ± SEM of 4 independent experiments were graphed. A one-way ANOVA with Bonferroni post hoc test (to correct of multiple comparisons) was used to assess statistical significance. Only γ-Pcdh-C3 significantly increased levels of Axin1. *p < 0.05 (b) Subcellular fractionation of HEK293 cell lysates overexpressing FL-C3GFP shows a significant shift in Axin1 localization to the membrane fraction in comparison cells expressing only the GFP control. The intensity of Axin1 bands from all membrane, cytoplasmic, and nuclear fractions were quantified across six individual experiments and analysed with a two-way ANOVA with Bonferroni post hoc test. *p < 0.05 (c,d) Cells transfected with either GFP or FL-C3GFP were exposed to DMEM (control), or Wnt3a for 4 or 24h before being lysed and immunoblotted for phosphorylated Lrp6 (S1490), total Lrp6 and GAPDH. Levels of phosphorylated Lrp6 (normalized to GAPDH) increase >2-fold in GFP-only transfected cells; this increase is significantly abrogated in the presence of g-Pcdh-C3 expression. The intensity of the phospho-Lrp6 band for each condition was normalized to GAPDH in the same lane, and means of 3 individual experiments ± SEM were graphed. A two-way ANOVA with Bonferroni post hoc test was performed. *p < 0.05 MW, molecular weight; kDa, kilodaltons.