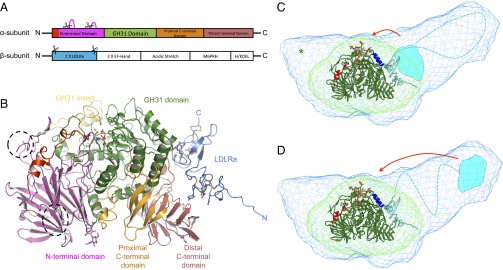
Fig. 1.
SAXS and crystal structures of Mmα-GluII. (A) A 1-Dimensional representation of the α-GluII subunits. The trypsinolysis sites are symbolized by scissors, and portions of the α-subunit removed by trypsin are represented by magenta loops. (B) Crystal structure of Mmα-GluIITryps in cartoon representation. The unique α-subunit N-terminal extension (residues 33–54) is shown in red, and the N-terminal domain (residues 55–392) is shown in purple. Dotted lines circle the α-subunit portions removed by trypsinolysis. The α-subunit GH31 catalytic domain (residues 393–748) is shown in green, with its insertion subdomain shown in yellow (residues 492–531). The proximal and distal α-subunit C-terminal domains (residues 749–828 and 829–966, respectively) are shown in orange and pink, respectively. After trypsinization, the α/β Mmα-GluII dimer retains the N-terminal domain of the β-subunit, adopting two tandem LDLRa folds (blue), with the trypsinized β-subunit N and C termini marked by a letter. Two calcium ions in the β-subunit LDLRa subdomains are shown as green spheres. The α-subunit active site residues, the N-linked glycan at N97, and the calcium-coordinating residues in the β-subunit are depicted in stick representation. (C and D) Crystal structure of Mmα-GluIITryps (in cartoon representation) overlaid on the SAXS-hydrated envelopes for Mmα-GluIITryps (green mesh) and Mmα-GluII (light blue mesh). The green cartoon illustrates the α-subunit and the cyan cartoon illustrates the β-subunit (N-terminal LDLRa domains), the dashed line illustrates the central part of the protein (unannotated), and the cyan shape illustrates the C-terminal M6PRH domain. The α-GluII–specific α-subunit N-terminal extension is painted red. The green asterisk illustrates the volume taken up by the residues α 186–240, removed from Mmα-GluIITryps by trypsinolysis. Helices α 427–441 and α 470–482, which the β-subunit protects from HDX, are shown in dark blue. A stereoregular, extended conformation of the Glc2Man9GlcNAc2 glycan, with the terminal Glc residues inserted in the active site, is represented in sticks (yellow, carbon atoms; red, oxygen atoms). Two possible locations of the β-subunit M6PRH domain (cyan shape) are shown, either proximal to the α-subunit active site (C) or distal to the α-subunit active site (D), at the far end of the light blue holoenzyme SAXS envelope. The red arrows indicate hypothetical conformational changes in the β-subunit that would bring its M6PRH domain closer to the enzyme’s α-subunit active site if simultaneous binding of both subunits to a single monoglucosylated glycan was necessary for cleavage.