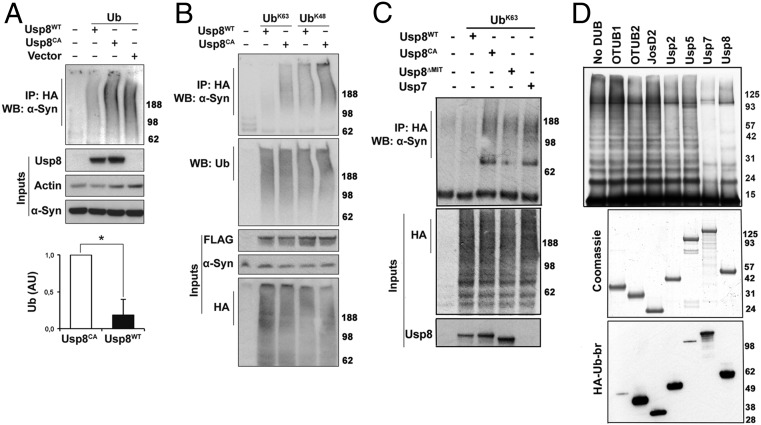
Fig. 4.
Usp8 deconjugates preferentially K63-linked ubiquitin chains on α-synuclein. (A) Expression of Usp8WT caused robust deubiquitination of endogenous α-synuclein compared with expression of Usp8CA or empty vector. Quantification of ubiquitinated α-synuclein in Usp8WT- relative to Usp8CA-expressing cells shown as arbitrary units (AU; *P = 0.0410, n = 3 biological replicates). WB, Western blot. (B) Coexpression of Usp8 with either K63 or K48 single-lysine ubiquitin showed that it deconjugated preferentially K63-linked chains on α-synuclein without any obvious difference in the total amount of ubiquitinated proteins between the immunoprecipitated samples (representative of n = 3 biological replicates). (C) Expression of a deletion mutant of Usp8 lacking the MIT domain required for endosomal localization (Usp8ΔMIT) reduced its activity against K63-linked ubiquitin chains on α-synuclein compared with Usp8WT. Usp7 had reduced activity against α-synuclein compared with Usp8 in cells (n = 5 biological replicates). (D) Recombinant α-synuclein was linked to uniform K63-linked chains in vitro by purified Nedd4 and incubated with the indicated deubiquitinases (50 nM). Robust deubiquitination was observed with Usp7 and Usp8. All seven enzymes were assessed for purity with Coomassie and activity by HA-Ub-Br labeling. Immunoblotting with anti-HA revealed a shift in the molecular mass of labeled DUBs, which indicates covalent binding to HA-Ub-Br. Error bars correspond to standard error of the mean.