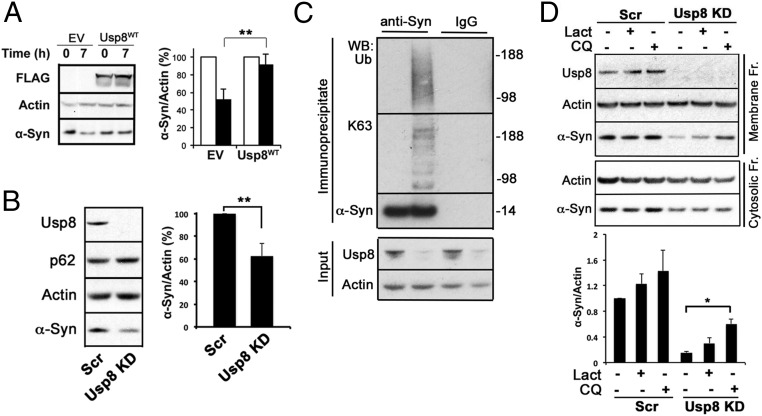
Fig. 5.
Usp8 regulates the degradation of α-synuclein by the lysosome. (A) Cycloheximide chase of endogenous α-synuclein showed that its rate of clearance was reduced at 7 h when wild-type Usp8 was expressed in HEK-293T cells (**P = 0.0091, n = 3 biological replicates). (B and C) Lentiviral-mediated shRNA knockdown of Usp8 in SH-SY5Y cells reduced endogenous α-synuclein levels by 35% relative to the actin loading control compared with Scr shRNA controls (**P = 0.0031, n = 5 biological replicates) (B) and increased the amount of ubiquitinated α-synuclein as evidenced by immunoblotting of immunoprecipitated α-synuclein with anti-ubiquitin and anti-K63 antibodies (C). No smear was seen when anti-HA was used as an IgG control. (D) Lysates isolated from Usp8 knockdown and Scr shRNA-treated SH-SY5Y cells were fractionated into cytosolic and membrane fractions and tested for α-synuclein levels at baseline and following 8 h of treatment with either 50 μM chloroquine (CQ) or 5 μM lactacystin (Lact). Accumulation of α-synuclein was observed in the membrane fraction, which is enriched in endosomal/autophagic–lysosomal compartments in Usp8 knockdown cells treated with chloroquine, suggesting that there is accelerated lysosomal degradation of α-synuclein in these cells (*P = 0.019, n = 3). Error bars correspond to standard error of the mean.