Supplemental Digital Content is Available in the Text.
Upper limb experimental heat pain is attenuated by lower limb nonpainful compression pants in an area-dependent mode, and in correlation with conditioned pain modulation, in healthy controls.
Keywords: Conditioned pain modulation, Mechanical compression, Heat pain, Lymphatic drainage therapy, Compression-induced analgesia
Abstract
Compression therapy, a well-recognized treatment for lymphoedema and venous disorders, pressurizes limbs and generates massive non-noxious afferent sensory barrages. The aim of this study was to study whether such afferent activity has an analgesic effect when applied on the lower limbs, hypothesizing that larger compression areas will induce stronger analgesic effects, and whether this effect correlates with conditioned pain modulation (CPM). Thirty young healthy subjects received painful heat and pressure stimuli (47°C for 30 seconds, forearm; 300 kPa for 15 seconds, wrist) before and during 3 compression protocols of either SMALL (up to ankles), MEDIUM (up to knees), or LARGE (up to hips) compression areas. Conditioned pain modulation (heat pain conditioned by noxious cold water) was tested before and after each compression protocol. The LARGE protocol induced more analgesia for heat than the SMALL protocol (P < 0.001). The analgesic effect interacted with gender (P = 0.015). The LARGE protocol was more efficient for females, whereas the MEDIUM protocol was more efficient for males. Pressure pain was reduced by all protocols (P < 0.001) with no differences between protocols and no gender effect. Conditioned pain modulation was more efficient than the compression-induced analgesia. For the LARGE protocol, precompression CPM efficiency positively correlated with compression-induced analgesia. Large body area compression exerts an area-dependent analgesic effect on experimental pain stimuli. The observed correlation with pain inhibition in response to robust non-noxious sensory stimulation may suggest that compression therapy shares similar mechanisms with inhibitory pain modulation assessed through CPM.
1. Introduction
Endogenous analgesia (EA) is a centrally mediated mechanism that inhibits pain at the spinal level by a “top–down” modulation system, based on a cortico–brain–stem–spinal pathway.21,55 Endogenous analgesia is based on several mechanisms that can exert pain inhibition. One of the most explored mechanisms of pain modulation is “diffuse noxious inhibitory controls” (DNIC), which represents the phenomenon of “bottom–up” activation of the EA system, mediated by activation of the spino–bulbo–spinal loop.7,8 The DNIC mechanism is tested psychophysically in humans in the laboratory using the conditioned pain modulation (CPM) paradigm. In this paradigm, an ascending noxious input from a remote (heterotopic or extrasegmental) body area (“conditioning stimulus” [CS]) exerts descending analgesia on the perception of another noxious stimulus (“test stimulus”).72 It is of importance to clarify that beyond CPM, there are several different physiological mechanisms that exert pain inhibition (eg, distraction from painful stimulus,68 stress-induced analgesia,10 and vagal activity–related analgesia27). These pain inhibitory mechanisms may either stand-alone or be complementary to each other.35,51
It has been postulated that 2 factors related to the CS contribute to the efficacy of the DNIC/CPM response: its painfulness and its area. Indeed, the results of many studies point to a moderate positive association between the level of pain induced by the CS and CPM efficiency.19,57,71 Nevertheless, others showed that painfulness of the conditioning stimuli is not a necessary condition for exerting attenuation of the test stimulus pain; inhibitory effects of non-noxious sensations, such as tonic heat,38 vibrotactile,44,64 gastric distention,11 and deep breathing,4 have been observed on the perception of various noxious stimuli (electrical stimulation, phasic heat stimuli, and heat pain thresholds).11,34,37,38 Furthermore, it is well established that a larger area for conditioning stimulation exerts stronger pain inhibition, probably because of the effect of spatial summation.12,36,47 Such a spatial summation effect was also reported for non-noxious sensation,28,34,46 and therefore may also be relevant for the pain-inhibiting effect of non-noxious “conditioning.”
Lymphatic drainage therapy (LDT), also known as compression therapy, is a well-recognized treatment for lymphoedema and venous disorders.9,29,70 Compression therapy systems include tubular bandages which are inflated to squeeze the limb, press the liquids up, improve venous return, and reduce edema. In addition to the main effect on the lymphatic system, application of increasing air pressure in the bandages results in massive non-noxious afferent sensory input that may theoretically exert EA. The aim of this study was to explore the effect of LDT-induced mechanical compression on the perception of experimental heat pain stimuli. We hypothesized that the compression will induce pain attenuation and that a larger size of the compression area will exert greater pain attenuation. In addition, we expected that the extent of compression-induced analgesia (CIA) will correlate with CPM efficiency.
2. Methods
2.1. Subjects
The study sample included 30 paid healthy right-handed volunteers (15 men and 15 women), ranging in age from 20 to 40 (mean ± SD: 26.5 ± 3.4 years). Subjects were mainly students recruited by advertisement. The Institutional Review Board of Rambam Health Care Campus approved the study protocol in accordance with the Declaration of Helsinki, and written consent was obtained from each subject before the beginning of the experiment. Participants were enrolled in the study after meeting the following criteria: (1) age above 18 and below 40; (2) absence of acute or chronic pain disorders; (3) no reports of psychiatric, cognitive, and/or neurological disorders; (4) no drugs including, analgesics or antianxiety medications on regular use (except for oral contraceptives); and (5) ability to give informed consent, communicate, and understand the purpose and the instructions of the study.
2.2. Instruments and stimulation parameters
(1) Mechanical compression of the lower limbs was applied by the Lympha Press Optimal® and Lympha Pants® (Mego Afek, Afek AC, Israel) mounted up to the greater trochanter of the femur. The pants contain 24 partially overlapping inflatable cells, which allow compression of different regions of the lower limb. The compression was applied with constant air pressure of 60 mm Hg which is less than the diastolic blood pressure in the area.
(2) The heat test stimulus was 30 second-long tonic heat delivered at fixed temperature of 47°C. As was shown by our studies, this temperature evoked moderate pain sensation.25,27 This was delivered by a contact-heat Thermal Sensory Analyzer 2001 system (Medoc, Ramat-Yishai, Israel), which delivered the heat stimulations by a 30 × 30 mm Peltier surface stimulator, attached by Velcro straps to the volar surface of the forearm of the subjects' left hand. Baseline temperature was 32.0°C, with an increasing temperature rate of 1°C/s and a return-to-baseline temperature rate of 8°C/s.
(3) The pressure test stimulus was 15 second-long tonic pressure delivered at the fixed intensity of 300 kPa. This stimulation intensity was chosen based on moderate pain scores obtained in our small pilot study. This was delivered by a Computerized Pressure Algometer (AlgoMed; Medoc) with a flat 1-cm2 circular probe covered with 1-mm thick rubber. The pressure was applied perpendicular to the dorsal aspect of the distal forearm (between the radius and ulna on the extensor retinaculum). The pressure was measured by kilopascal units. Baseline pressure was 0 kPa, with an increasing rate of 50 kPa/s.
(4) The CS was 65 second-long water immersion. This was delivered by a plastic container, 39 × 28 × 14 cm, with cold water maintained at 8 to 10°C. The temperature was maintained by repeatedly adding ice and constantly monitored by a thermometer. The right foot of the subjects was immersed up to the level of the ankle.
Pain ratings of heat and pressure stimuli were assessed continuously by the computerized visual analog scale (COVAS), whereas pain intensity ratings from the CS were assessed verbally using numerical pain scale (NPS). Both scales are ranging from 0, denoting “no pain,” to 100, denoting “the worst imaginable pain.” Pain ratings to CS were obtained 10 and 20 seconds after the initiation and at the end of the CS (65 seconds).
2.3. Training session
At the beginning of the experimental session, all subjects underwent short training to familiarize them with the devices and with the sensations evoked by the various painful stimulation modalities. They were trained to report their perceived pain intensity using the NPS and the COVAS. The training included the following:
(1) Exposure to 3 short contact-heat stimuli (43, 45, and 47°C), each lasting for 7 seconds from the time that the stimulation intensity reached the destination temperature. The thermode was moved following each stimulus to a completely distinct adjacent area of skin.
(2) Exposure to 2 short pressure stimuli (150 and 400 kPa), each lasting for 7 seconds from the time that the stimulation intensity reached the destination pressure. Subjects were asked to rate the level of pain intensity induced by each stimulation using the COVAS.
(3) Exposure to cold water by immersing their foot in the container for 10 seconds. They were asked to rate the level of pain intensity by NPS at the end of the immersion.
2.4. Experiment protocol
All experiments were conducted in the same setting by 2 investigators. One investigator (O.B.-B.) managed the area of compression, and the other (L.H.) blinded to the area of compression and managed the sensory assessments. The test area was hidden from view by means of a stand with curtain that covered the subject from the chest downwards. The experiment was performed in a quiet room with an ambient temperature of 23°C. The pre- and post-compression assessments were performed in seated position. The full protocol is presented in Figure 1. After the familiarization stage, the experiment begun with delivery of heat and pressure baseline (precompression) “test stimuli” performed in a pseudo-random order. After a 10-minute break, as part of the standard CPM paradigm, subjects immersed their right foot into the cold water bath (the CS) for 65 seconds; at the 30th second, a repeat heat test stimulus was delivered. Note that the CPM response was assessed for the heat test stimulus only. Ten minutes after the baseline precompression CPM assessment, subjects were tested under 3 compression protocols while lying down, delivered by Lympha pants, each lasting 12 minutes after achieving the destination pressure. The compression protocols were performed in a random sequential order, in one session. In each protocol, the sustained compression was delivered to different areas of the limbs: (1) up to the ankles (SMALL), (2) up to the knees (MEDIUM), and (3) up to the hip joints (LARGE). During each compression protocol, the investigators confirmed with the subjects that the compression did not cause any pain or discomfort. In case of discomfort, the body position was changed until no longer uncomfortable. All participants received an explanation regarding the approximate duration of the compression protocol; no indications or suggestions were made regarding expected CIA.
Figure 1.
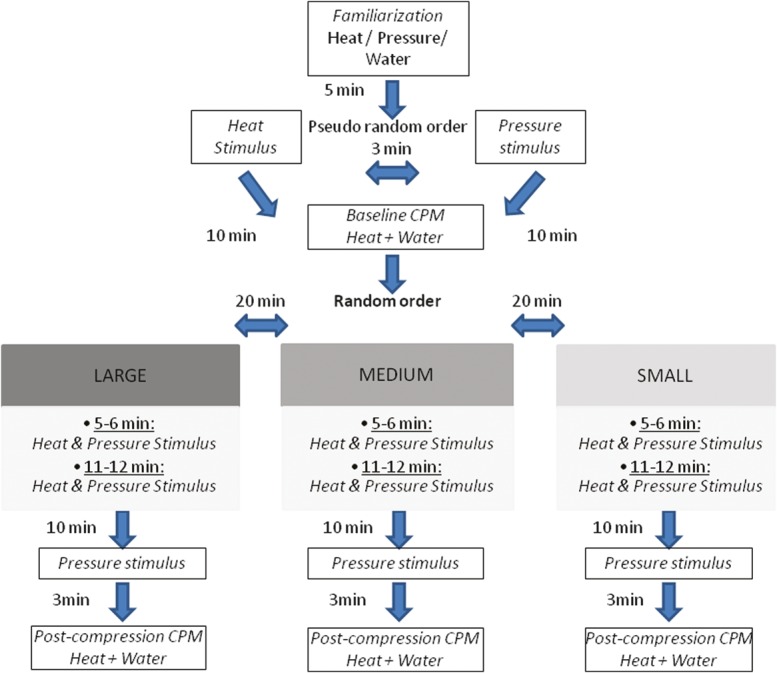
Schematic representation of the experimental design. CPM, conditioned pain modulation; SMALL, compression area up to the ankle; MEDIUM, compression area up to the knees; LARGE, compression area up to the hip joint.
Heat and pressure test stimuli were delivered sequentially during each compression protocol in the same order and manner as the baseline test stimulations, twice: at 5 to 6 and 11 to 12 minutes of the compression. Analgesic effect of each compression protocol was assessed as a difference between the heat or pressure pain scores obtained during the compression vs baseline (the CIA responses).
To evaluate possible residual analgesic effect, the pressure pain sensitivity was tested at 10 minutes after the completion of each compression protocol (Fig. 1). Following the postcompression pressure pain assessment, heat pain test stimulus combined with CS was delivered, and pain scores were obtained. The baseline CPM effect was calculated as the difference in mean pain ratings of the heat test stimulus delivered under CS vs the heat test stimulus delivered stand-alone. For the postcompression conditions, the CPM was calculated as the difference between the pain scores to heat test stimulus delivered under CS from precompression heat pain “test stimulus.”
Negative values indicate efficient CPM or efficient CIA.
Twenty-minute rest intervals were provided in between each of the 3 compression protocols. The total duration of the experimental session was approximately 2 hours and 15 minutes.
2.5. Data analysis
Data were processed and analyzed using Excel (Microsoft Corp, Redmond, WA) and JMP (SAS Institute, Cary, NC) software. The results are expressed as mean ± SE, unless mentioned otherwise.
Two separate repeated-measures analyses of variance (rmANOVAs, using mixed models) were performed on the data. The first was a one-way model comparing the pain scores with the baseline precompression test stimulus pain scores with the pain scores of the test stimuli delivered under each compression protocol (termed SMALL, MEDIUM, and LARGE, based on the area of applied compression) at 2 time points of assessment (T1: 5-6 minutes; T2: 11-12 minutes), for males and females. This was to determine, by post hoc Tukey tests, whether there was any pain reduction at each individual time and protocol relative to baseline. The second model evaluated the effect of the compression protocols, the time points of test stimulus pain assessment, and the effect of gender and their interaction on the changes in pain perception relatively to the baseline pain scores (difference scores). Although this might have been accomplished with appropriate contrasts to the previous analysis, this analysis uses a simpler design to determine whether there are differences in pain reduction, with specific regard to time, compression protocol, and their interaction.
In addition, we also analyzed the postcompression CPM and pressure pain score data using 2 methods. Repeated-measures ANOVA (mixed model) used the compression protocol (baseline, SMALL, MEDIUM, and LARGE), gender, and their interaction. A second analysis model used difference scores of CPM and pressure pain relative to baseline. Repeated-measures ANOVA (mixed model) used the compression protocol (SMALL, MEDIUM, and LARGE), gender, and their interaction.
Pearson correlations were used to explore possible relations between the analgesic effect of each one of the compression protocols and (1) the pain ratings to the test stimulus under CS at baseline and (2) baseline CPM effect. In addition, the correlation analysis was applied for the CPM responses tested pre- and post-compression. Statistical significance was defined as P ≤ 0.05.
3. Results
3.1. Subject subgroups based on test stimuli pain reports
The main aim of the study was to explore the analgesic effect of mild compression on painful experimental stimuli. During the data analysis, we noticed that some subjects experienced only mild pain from the “test stimuli.” We therefore decided to report two sub-sets of analyses: for the whole group, and; for the sub-group of subjects whose mean COVAS score to the baseline test-stimuli was >20. Based on previous experience of our lab and other labs, pain scores <20 are considered as too mild a pain experience for inclusion in analyses.3,26,73 Using this cutoff, we aimed to eliminate the possible floor effect on the precompression pain scores.
No subjects reported pain due to any compression protocol.
3.2. Compression-induced analgesia effect on experimental heat pain
For the whole group, a significant effect of the compression protocol (P = 0.017), with no effect of gender (P = 0.776), or significant compression protocol X gender interaction (P = 0.062). Overall, the heat pain scores decreased significantly following the LARGE protocol from 38.6 ± 2.9 (baseline) to 31.8 ± 2.9 at T1 (P = 0.013) and to 32.2 ± 2.9 at T2 (P = 0.023). Nonsignificant decrease from the baseline was observed for SMALL (35.4 ± 2.9, P = 0.689, T1 and 35.0 ± 2.9, P = 0.543, T2) and MEDIUM protocols (33.6 ± 2.9, P = 0.153, T1 and 34.3 ± 2.9, P = 0.307, T2). The males and females were no different in their baseline heat pain perception (43.8 ± 4.5 and 44.3 ± 4.1, respectively, P = 0.1). Males and females were also of same age: 26.5 ± 2.8 (mean ± SD) and 26.4 ± 4.0 years, respectively.
For the group of subjects with the mean heat pain scores >20, N = 24 (26.2 ± 3.0 years, 13 females), significant effect of the compression protocol (P < 0.001) was in interaction with gender (P = 0.015), rmANOVA. More specifically, the heat pain scores assessed at both time points during the LARGE compression protocol were lower than the baseline pain scores (P = 0.001 for T1 and P = 0.002 for T2). In addition, pain assessed at T1 during the MEDIUM compression protocol was also significantly lower than the pain at baseline (P = 0.005), Figure 2A. The interaction with gender was based on the lower pain scores during the MEDIUM protocol in males (P = 0.030, T1) and during the LARGE protocol in females (P = 0.050, T2), Figure 2B.
Figure 2.
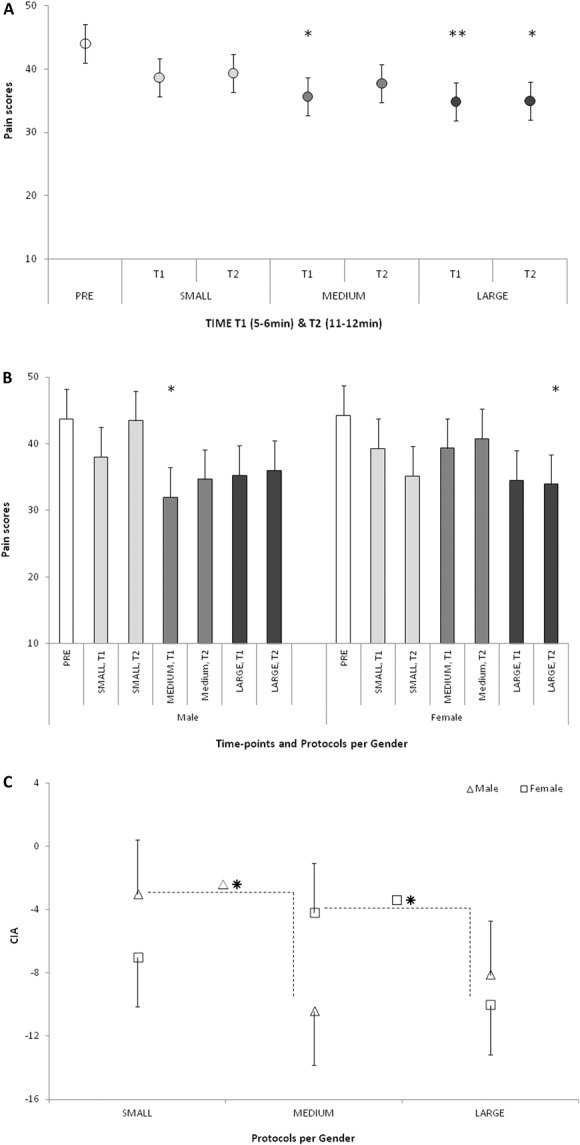
The CIA effect on heat pain scores in 3 compression protocols. (A) Overall, significant pain reduction from the precompression baseline was observed during MEDIUM protocol at T1, and during LARGE protocol at T1 and T2. (B) For males, the maximal pain reduction from the baseline was observed at T1 during MEDIUM protocol; for females, the maximal pain reduction was observed at T2 during LARGE protocol. (C) In males, the MEDIUM protocol induced more efficient CIA than the SMALL protocol; in females, the LARGE protocol induced more efficient CIA than the SMALL protocol. *P ≤ 0.05; **P ≤ 0.01. CIA, compression-induced analgesia; SMALL, compression area up to the ankle; MEDIUM, compression area up to the knees; LARGE, compression area up to the hip joint.
Second rmANOVA model indicated that greater extent of pain reduction relative to baseline (P = 0.025) was due to the LARGE as compared with the SMALL protocols (P = 0.019), with no effect of the assessment time points (P = 0.434). Significant interaction between the stimulation protocol and gender pointed to greater analgesic effect of the MEDIUM as compared with the SMALL protocol in males (P = 0.013), and of the LARGE as compared with the MEDIUM protocol in females (P = 0.047), Figure 2C.
3.3. Compression-induced analgesia effect on experimental pressure pain
For the whole group, there was neither a significant effect of the compression protocol at either time (P = 0.332) nor effect of gender (P = 0.106), or compression protocol at either time X gender interaction (P = 0.855). Across all conditions, the pressure pain scores were in the range between 17.9 ± 3.1 and 24.9 ± 3.1. The males and females were no different in their baseline pressure pain perception (21.1 ± 4.4 vs 28.6 ± 4.4, respectively, P = 0.277).
For the group of subjects with the mean pressure pain scores >20, N = 17 (26.9 ± 3.9 years, mean ± SD, 10 females), there was a significant effect of the stimulation protocol at either time (P = 0.010) with no effect of gender (P = 0.592), or compression protocol at either time X gender interaction (P = 0.833). The post hoc analysis revealed that except the T2 time point of LARGE protocol stimulation, the pressure pain scores under all compression protocols were lower than those assessed at baseline (Fig. 3A), with no difference between males and females (Fig. 3B).
Figure 3.
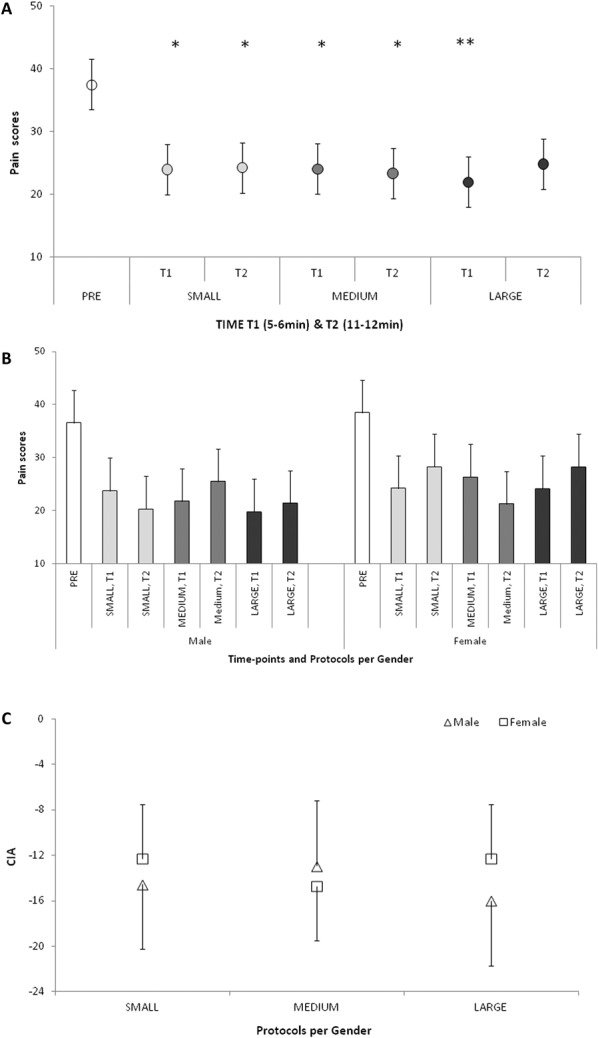
The CIA effect on the pressure pain scores in 3 compression protocol. (A) Overall, significant pain reduction from the precompression baseline was observed during all compression protocols at both T1 and T2 time points, except the pain scores at T2 during the LARGE protocol. (B) No gender differences were observed for the maximal pain reduction. (C) No differences in the efficiency of 3 compression protocols between males and females. *P ≤ 0.05; **P ≤ 0.01. CIA, compression-induced analgesia; SMALL, compression area up to the ankle; MEDIUM, compression area up to the knees; LARGE, compression area up to the hip joint.
As shown by the ANOVA on pain reduction, no difference in the extent of pressure pain reduction across the compression protocols, relative to baseline, was observed (P = 0.857), with no effect of gender (P = 0.835), or assessment time points (P = 0.724), Figure 3C.
In addition, we tested the postcompression effect on the pressure pain scores in the group of 17 subjects. The results were similar to those obtained during the compression: lower pain scores obtained 10 minutes after completing each compression protocol (P < 0.001), with no effect of gender (P = 0.704), or compression protocol X gender interaction (P = 0.228). The second rmANOVA model confirmed that there was no difference in extent of pressure pain reduction between the compression protocols (P = 0.545), with no interaction with gender (P = 0.112).
3.4. Effect of the compression area on conditioned pain modulation efficiency
The CPM response was calculated for subjects whose pain scores to the test stimulus was >20.
There was a significant effect of the stimulation protocol (P = 0.032) that was in interaction with gender (P = 0.025). The post hoc analysis indicated that CPM assessed after MEDIUM protocol was more efficient that after the SMALL protocol (P = 0.019) (Fig. 4A), with no difference between males and females. The interaction with gender was based on the lower CPM response after the SMALL protocol in males as compared with the baseline (P = 0.041) (Fig. 4B).
Figure 4.
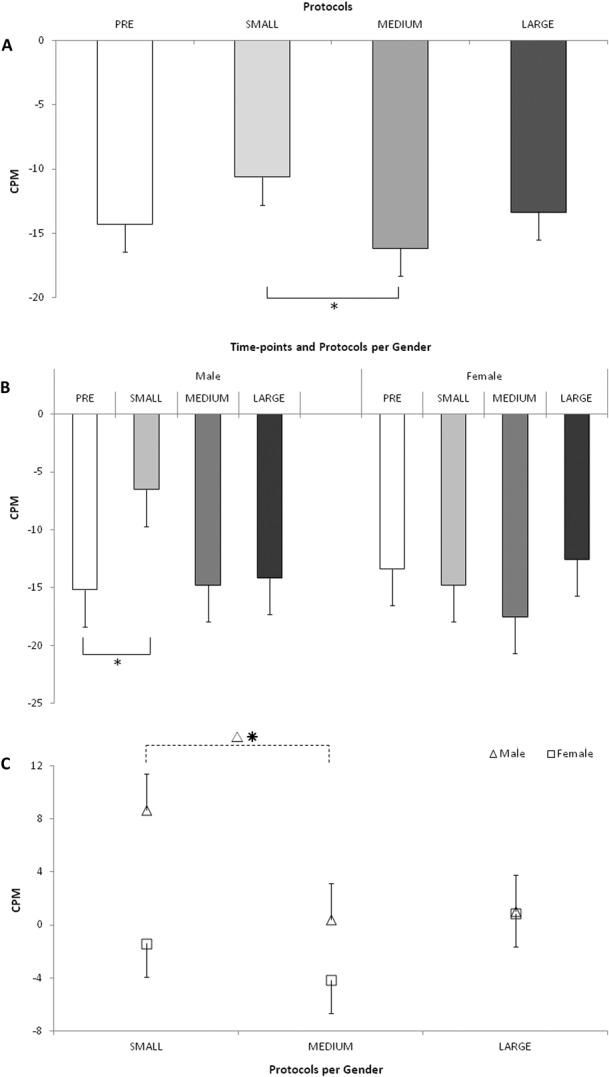
The CPM response at the precompression baseline and after 3 compression protocols. (A) Overall, no significant difference was found between the pre- and post-compression CPM assessment. However, the CPM was more efficient after the MEDIUM protocol as compared with the SMALL protocol. (B) For males, lower CPM efficiency was observed following the SMALL as compared with the MEDIUM compression protocols; for females, similar CPM responses were denoted across all conditions. (C) For males, the CPM efficiency was reduced from the baseline following the SMALL as compared with the changes in CPM efficiency following the MEDIUM protocol. *P ≤ 0.05. CPM, conditioned pain modulation; SMALL, compression area up to the ankle; MEDIUM, compression area up to the knees; LARGE, compression area up to the hip joint.
The second rmANOVA model indicated that the changes in the CPM response after exposure to the compression protocols (P = 0.015) were due to the increased CPM efficiency after the MEDIUM protocol and reduced CPM efficiency after SMALL protocol (P = 0.011), relative to baseline. This effect was mainly attributed to males but not females (P = 0.038; compression protocol X gender interaction P = 0.032), Figure 4C.
The pain scores of the CS were no different across the baseline and postcompression CPM assessments (45.8 ± 3.7 at baseline, 49.0 ± 3.7 after the SMALL protocol, 47.7 ± 3.7 after the MEDIUM protocol, and 48.5 ± 3.7 after the LARGE protocol; P = 0.203). A trend for overall higher pain scores was observed for females (54.1 ± 5 vs 41.5 ± 5, P = 0.088); however, this borderline effect of gender was not in interaction with the stimulation protocol (P = 0.855).
All subjects rated the baseline water pain >20 on NPS.
3.5. Comparison between conditioned pain modulation and compression-induced analgesia (N = 24)
Baseline CPM was more efficient than each of the CIA protocols (averaging across CIA at T1 and T2, P < 0.001). More specifically, baseline CPM (−14.3 ± 2.3) was more efficient than the CIA for SMALL (−5.0 ± 2.3, P < 0.001) and for MEDIUM compression protocols (−7.3 ± 2.3, P = 0.004). A borderline difference was found between the CPM and the CIA for LARGE protocol (−9.1 ± 2.3, P = 0.055). No effect of gender or gender interaction was observed.
Positive correlation was found between the heat pain scores obtained during foot water immersion (the conditioned test stimulus) and the heat pain scores obtained during compression. This was demonstrated for each protocol at each time point (T1 and T2); LARGE (r = 0.64, P < 0.001; r = 0.38, P = 0.001, respectively), MEDIUM (r = 0.37, P = 0.001; r = 0.38, P = 0.001, respectively), and SMALL (r = 0.46, P < 0.001; r = 0.46, P < 0.001, respectively) (Fig. 5). Furthermore, a positive correlation was observed between the precompression CPM efficiency and the CIA at both time points in the LARGE stimulation protocol (r = 0.42, P ≤ 0.001 at T1; r = 0.24, P = 0.014 at T2) (Fig. 6).
Figure 5.
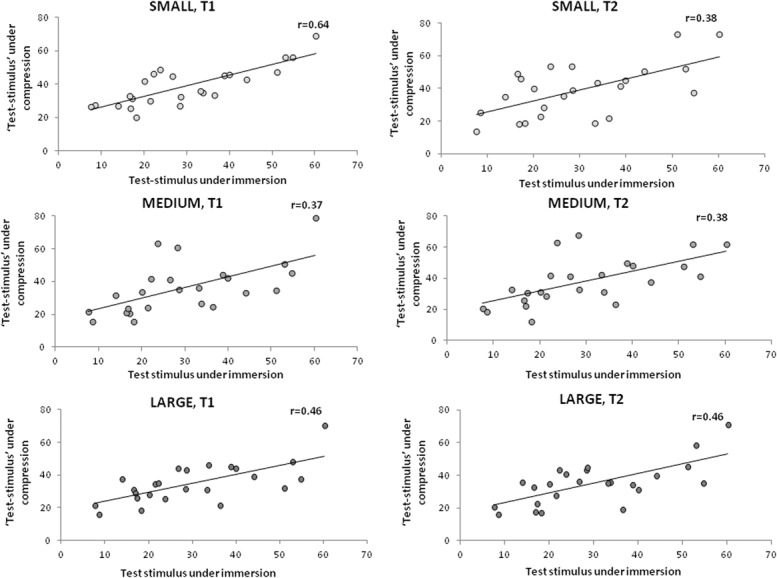
Relationship between the heat pain scores assessed under immersion at baseline and during each compression protocol. Significant correlations were found between the pain scores to test stimulus conditioned to water immersion, and heat pain scores to the test stimulus assessed during each of the compression protocols, at both testing time points. All P ≤ 0.001. SMALL, compression area up to the ankle; MEDIUM, compression area up to the knees; LARGE, compression area up to the hip joint.
Figure 6.
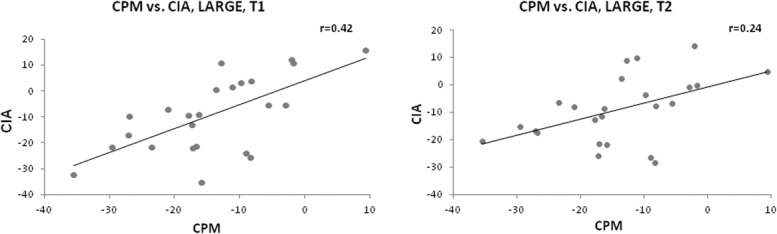
Relationships between CPM and CIA. Significant correlations were found between the baseline precompression CPM response and the CIA obtained during the LARGE protocol at both testing time points. All P ≤ 0.01. CIA, compression-induced analgesia; CPM, conditioned pain modulation; LARGE, compression area up to the hip joint.
3.6. Spread of pain scores to the heat test stimuli under different conditions
In addition to the presented statistical analyses, we performed a descriptive analysis of the number of subjects that showed facilitation during different conditioning stimuli (water immersion or compression). Among 24 subjects, 1 subject had heat pain facilitation during the water immersion, meaning nonefficient CPM response; 9 subjects demonstrated the pain facilitation (nonefficient CIA) during the SMALL protocol, 7 subjects demonstrated nonefficient CIA during the MEDIUM protocol, and 6 subjects demonstrated nonefficient CIA during the LARGE protocol. Same subject who had nonefficient CPM demonstrated also nonefficient CIA during 3 compression protocols (see Fig. 1 in the Supplementary Materials, available online at http://links.lww.com/PAIN/A274). Despite the efficient CPM, 5 subjects had nonefficient CIA during 3 compression protocols.
4. Discussion
To the best of our knowledge, this is the first study to investigate the analgesic effect of LTD-evoked mechanical compression. The main finding of our study is that mild mechanical compression applied to the lower body reduces perception of remote experimental pain stimuli. Furthermore, this analgesic effect was larger for larger stimulation area (the compression up to the thigh vs ankle), thus confirming the contribution of spatial summation of non painful conditioning stimulation to pain attenuation. Furthermore, the CIA was positively correlated with the CPM effect, which may suggest that conditioned pain modulation and compression-induced inhibitory pain modulation share similar physiological mechanisms.
The analgesic effect of mild body compression is expected. There are many reports showing that non-noxious somatosensory stimuli exert inhibitory pain modulation; this was demonstrated for various sensations, including tonic heat and vibrotactile.38,44,64 In line, some clinical procedures that involve nonpainful sensory stimulation, such as acupuncture,39,40,65 TENS,5,15,17,41,56 and hydrotherapy,2,16,22,48 are applicable for pain treatment. Pain reduction evoked by non-noxious somatosensory stimulation was observed not only in the psychophysical level but also in the neurophysiological level. For example, light touch reduced amplitudes of cortical pain-evoked potentials reflecting decreased activity in the anterior cingulate cortices and primary somatosensory cortex.44 Furthermore, brain-imaging studies showed that analgesic effect of electroacupuncture was associated with suppressive effect on the brain structures associated with the medial pain system (secondary somatosensory, cingulate and inferior frontal cortices, and amygdala) and involved in pain perception and processing. This suppression counteracts or minimizes the subsequent C-fiber–induced neuronal excitation mediated activation through the spinothalamic and spinopontoamygdaloid pain pathways.63,65
4.1. Mechanism underlying the analgesic effect of large-body compression
Compression-induced analgesia could arise from various underlying mechanisms, alone or complementary to each other, which may comprise different elements in the pain modulatory pathways:
(1) The large body area compression stimulus applied by air pressure may have an analgesic effect due to massive afferent sensory input, as was suggested by Wall and Melzack in their “Gate Control Theory of Pain”49,69; in which activity of large fibers (Aβ) exerts an inhibitory effect by supraspinal circuits on the small fibers (C), by specific circuitry in the dorsal horn, thus reducing pain. This theory may also explain the neurophysiologic and psychophysical effect of touch-induced analgesia in humans by inhibiting the activity of Aδ and C fibers.40,44 It was suggested that the latter is mediated by a spinal/subcortical gating of the ascending nociceptive input, which sequentially results in the modulation of supraspinal cortical responses.30,44,53 Importantly, wide dynamic range neurons within the spinal dorsal horn, which may be affected by DNIC, receive convergent input from both mechano receptive Aβ fibers and nociceptive Aδ and C fibers.13 As such, the dorsal horn integrates ascending, descending, and intraspinal signals and may act as a modulator in the inhibition process.66
(2) The autonomic nervous system (ANS) may be another key player. Pain regulation is an integral component of the sympathetic and the parasympathetic systems. Parasympathetic activation induces pain inhibition, as demonstrated, for example, in the study of Sedan62 in which parasympathetic activation by nonpainful gastric distension attenuated experimental pain response in healthy subjects, probably by gastric vagal afferents. In turn, reduced parasympathetic activity due to reduced vagal function in chemotherapy-induced neuropathy patients correlated with enhanced experimental pain.52 Lymphatic drainage has significant effects on the ANS, expressed by dampening sympathetic activity while increasing parasympathetic activity and manifested by increase in heart rate variance and cardiac parasympathetic activity in normal subjects.33 Venous velocity increases by 2- to 4-fold by the electronic LD device18,32,74 along with an increase in intrathoracic pressure, blood pressure, and venous return.67 These changes activate arterial and cardiopulmonary baroreceptors, sensitive to increased blood pressure and volume, which in turn activates the endorphins-rich brainstem area at the vagal nerve nucleus, nucleus tractus solitarious, which allow interactions between cardiovascular and pain regulatory systems during respiration.14 Indeed, activation of the nucleus tractus solitarious by baroreceptors decreases the vagal output to the heart and activates pain inhibitory mechanisms.14,59
(3) It is well established that excitability in the primary motor cortex (M1) is important for pain inhibition; enhancement of M1 excitability, for example by repeated transcranial magnetic stimulation, influences the nociceptive processing exerted directly at thalamic and spinal levels, and indirectly by activation of limbic, cortical, and subcortical structures associated with inhibitory pain modulation.20,43,54 Increase in M1 excitability was also reported in response to large-area non-noxious somatosensory stimuli. This was demonstrated by reduced motor activity threshold, increased amplitude of motor-evoked potentials, and increased intracortical facilitation in response to whole-hand or whole-body water flow stimulation60,61 and in response to whole-hand mesh-flow electrical stimulation.23,24 Thus, the theoretical increase in M1 excitability in large-body compression can be attributed to CIA in our study.
(4) Attentional distraction affects the perception of painful stimuli.42,51 We assume that distraction may be part of the CIA in our study because every compression protocol exercised pain reduction to some extent. However, because of significant effect of the compression area (LARGE vs SMALL) on pain reduction, we suggest that cognitive attention recruitment in response to a CS has a small role in our results.
(5) The conditioned pain modulation is one of the relevant physiological mechanisms that may explain our findings. Because DNIC influence wide dynamic range neurons, which are activated by nonpainful mechanical stimulation, it is reasonable to assume that the analgesic effect may occur because of the DNIC phenomena, given that the compression stimulus was not painful. The pre-eminent factor that influences both DNIC and CPM efficiency is the size of the CS. Several studies on both animals and humans have revealed that nociceptive activity increases concomitantly with the size of the surface area stimulated up to a certain point, after which its activity gradually decreases regardless of the continued augmentation in the size of the surface area stimulated.12,45 These experiments demonstrate that spatial summation triggers inhibitory feedback mechanisms onto multireceptive neurons when the stimulated surface is 2-3 times larger than the initially stimulated area.6,12 These results can be applicable to our findings on pain modulation by non-noxious mechanical compression when larger compression area exerted stronger heat pain reduction compared with the small compression area. Interestingly, the CIA effect on perceived pain was also detected 10 minutes after the end of compression. The poststimulation analgesic effect is not entirely surprising; as it is well known that the effect of DNIC can last for tens of minutes, depending on the stimulation paradigm.58 In our case, the conditioning stimulation is more robust than in most studies reported, encompassing very large body areas, so this might contribute to its persistence. Additional evidence for similarity between CIA and CPM is that the pain perception of the test stimulus under cold-water immersion positively correlated with the test stimulus under compression, for all 3 compression protocols. Importantly, only for the LARGE compression protocol, the CPM was significantly correlated with CIA, additionally highlighting the role of stimulated area in producing pain inhibition.
Despite that, in general, the CPM was more efficient than the CIA, different compression protocols induced different CIA in males and females for heat pain. The greater analgesic effect in males was obtained in the MEDIUM protocol, whereas females had greater analgesic effect in the LARGE protocol. These findings can be attributed to greater muscle mass in males.1,31,50 It is reasonable therefore to assume that the relatively low pressure used in the compression (60 mm Hg) was not enough to press the quadriceps muscle against the deep vein circumflex associated with the femur in males; whereas the same pressure was sufficient for females, thus reducing pain through the ANS for the MEDIUM compression protocol.
Furthermore, among the 3 compression-induced protocols, the more efficient CPM response was obtained after the MEDIUM compression protocol, and less efficient after the SMALL protocol. Because CPM was tested not during but minutes after completing compression, we suggest that stronger analgesic effect induced by the LARGE protocol reached a ceiling effect, such that no further pain reduction was obtained in the subsequent CPM assessment. In line with this explanation, the MEDIUM protocol that was less efficient in term of CIA, therefore, might have induced some additive effect on the consequently tested CPM response, explaining greater CPM efficiency after the MEDIUM compression protocol. Reduction of the CPM efficiency after the SMALL protocol was gender related and found in males. This finding is hard to explain. We may suggest that lower pain scores in males to foot immersion in cold noxious water (a CS) may have negative additive effect to SMALL compression protocol directed to the feet.
Our main study limitation is a small sample size for subjects who experienced pressure pain in baseline. This may be the reason for the dissimilarity between CIA on heat as compared to pressure pain; in contrast to its effect on heat pain, the CIA effect on pressure pain was similar for all applied compression protocols. Future study on larger samples is needed to conclude whether the CIA effect is modality dependent.
5. Conclusions
This study is the first to demonstrate that large-body area mild LDT compression exerts an analgesic effect on experimental pain stimuli. We suggest that CIA shares similar mechanisms with various facets of inhibitory pain modulation, when the mechanism of CPM seems to us a main contributing factor. We suggest, therefore, that compression therapy may be potentially used for pain treatment.
Conflict of interest statement
Y. Granovsky is a pain consultant of Medoc Ltd; she also was a paid consultant to Mego Afek during 2014. E. Sprecher has consulted for Medoc Ltd in the past. The other authors have no conflicts of interest to declare.
This study was sponsored by Mego Afek AC, Afek, Israel.
Supplementary Material
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A274.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Abe T, Kearns CF, Fukunaga T. Sex differences in whole body skeletal muscle mass measured by magnetic resonance imaging and its distribution in young Japanese adults. Br J Sports Med 2003;37:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahern M, Nicholls E, Simionato E, Clark M, Bond M. Clinical and psychological effects of hydrotherapy in rheumatic diseases. Clin. Rehabil. 1995;9:204–12. [Google Scholar]
- [3].Albu S, Gómez-Soriano J, Avila-Martin G, Taylor J. Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. PAIN 2015;156:260–72. [DOI] [PubMed] [Google Scholar]
- [4].Arsenault M, Ladouceur A, Lehmann A, Rainville P, Piché M. Pain modulation induced by respiration: phase and frequency effects. Neuroscience 2013;252:501–11. [DOI] [PubMed] [Google Scholar]
- [5].Aubin M, Marks R. The efficacy of short-term treatment with transcutaneous electrical nerve stimulation for Osteo-arthritic knee pain. Physiotherapy 1995;81:669–75. [Google Scholar]
- [6].Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res 2002;40:29–44. [DOI] [PubMed] [Google Scholar]
- [7].Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. PAIN 1979;6:283–304. [DOI] [PubMed] [Google Scholar]
- [8].Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. PAIN 1979;6:305–27. [DOI] [PubMed] [Google Scholar]
- [9].Bergan JJ, Sparks S, Angle N. A comparison of compression Pumps in the treatment of lymphedema. Vasc Endovasc Surg 1998;32:455–62. [Google Scholar]
- [10].Bodnar RJ, Kelly DD, Brutus M, Glusman M. Stress-induced analgesia: neural and hormonal determinants. Neurosci Biobehav Rev 1980;4:87–100. [DOI] [PubMed] [Google Scholar]
- [11].Bouhassira D, Chollet R, Coffin B, Lémann M, Le Bars D, Willer JC, Jian R. Inhibition of a somatic nociceptive reflex by gastric distension in humans. Gastroenterology 1994;107:985–92. [DOI] [PubMed] [Google Scholar]
- [12].Bouhassira D, Gall O, Chitour D, Le Bars D. Dorsal horn convergent neurones: negative feedback triggered by spatial summation of nociceptive afferents. PAIN 1995;62:195–200. [DOI] [PubMed] [Google Scholar]
- [13].Bromm B, Lorenz J. Neurophysiological evaluation of pain. Electroencephalogr Clin Neurophysiol 1998;107:227–53. [DOI] [PubMed] [Google Scholar]
- [14].Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev 2004;28:395–414. [DOI] [PubMed] [Google Scholar]
- [15].DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep 2008;10:492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dundar U, Solak O, Yigit I, Evcik D, Kavuncu V. Clinical effectiveness of aquatic exercise to treat chronic low back pain: a randomized controlled trial. Spine (Phila Pa 1976) 2009;34:1436–40. [DOI] [PubMed] [Google Scholar]
- [17].Emmiler M, Solak O, Kocogullari C, Dundar U, Ayva E, Ela Y, Cekirdekci A, Kavuncu V. Control of acute postoperative pain by transcutaneous electrical nerve stimulation after open cardiac operations: a randomized placebo-controlled prospective study. Heart Surg Forum 2008;11:E300–3. [DOI] [PubMed] [Google Scholar]
- [18].Flam E, Berry S, Coyle A, Dardik H, Raab L, Brunswick E. Blood-flow augmentation of intermittent pneumatic compression systems used for the prevention of deep vein thrombosis prior to surgery. Am J Surg 1996;171:312–15. [DOI] [PubMed] [Google Scholar]
- [19].Fujii K, Motohashi K, Umino M. Heterotopic ischemic pain attenuates somatosensory evoked potentials induced by electrical tooth stimulation: diffuse noxious inhibitory controls in the trigeminal nerve territory. Eur J Pain 2006;10:495–504. [DOI] [PubMed] [Google Scholar]
- [20].Garcia-larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage 2007;37:71–9. [DOI] [PubMed] [Google Scholar]
- [21].Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev 2004;27:729–37. [DOI] [PubMed] [Google Scholar]
- [22].Geytenbeek J. Evidence for effective hydrotherapy. Physiotherapy 2002;88:514–29. [Google Scholar]
- [23].Golaszewski SM, Bergmann J, Christova M, Kunz AB, Kronbichler M, Rafolt D, Gallasch E, Staffen W, Trinka E, Nardone R. Clinical Neurophysiology Modulation of motor cortex excitability by different levels of whole-hand afferent electrical stimulation. Clin Neurophysiol 2012;123:193–9. [DOI] [PubMed] [Google Scholar]
- [24].Golaszewski SM, Bergmann J, Christova M, Nardone R, Kronbichler M, Rafolt D, Gallasch E, Staffen W, Ladurner G, Beisteiner R. Clinical Neurophysiology Increased motor cortical excitability after whole-hand electrical stimulation: a TMS study. Clin Neurophysiol 2010;121:248–54. [DOI] [PubMed] [Google Scholar]
- [25].Granot M, Granovsky Y, Sprecher E, Nir RR, Yarnitsky D. Contact heat-evoked temporal summation: tonic versus repetitive-phasic stimulation. PAIN 2006;122:295–305. [DOI] [PubMed] [Google Scholar]
- [26].Granot M, Weissman-fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? PAIN 2008;136:142–9. [DOI] [PubMed] [Google Scholar]
- [27].Granovsky Y, Miller-Barmak A, Goldstein O, Sprecher E, Yarnitsky D. CPM test-Retest Reliability: “standard” vs “Single test-stimulus” protocols. Pain Med 2016;17:521–29. [DOI] [PubMed] [Google Scholar]
- [28].Green BG, Zaharchuk R. Spatial variation in sensitivity as a factor in measurements of spatial summation of warmth and cold. Somatosens Mot Res 2001;18:181–90. [DOI] [PubMed] [Google Scholar]
- [29].Hassall A, Graveline C, Hilliard P. A retrospective study of the effects of the Lymphapress pump on lymphedema in a pediatric population. Lymphology 2001;34:156–65. [PubMed] [Google Scholar]
- [30].Inui K, Tsuji T, Kakigi R. Temporal analysis of cortical mechanisms for pain relief by tactile stimuli in humans. Cereb Cortex 2006:355–65. [DOI] [PubMed] [Google Scholar]
- [31].Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985) 2000;89:81–8. [DOI] [PubMed] [Google Scholar]
- [32].Killewich LA, Sandager GP, Nguyen AH, Lilly MP, Flinn WR. Venous hemodynamics during impulse foot pumping. J Vasc Surg 1995;22:598–605. [DOI] [PubMed] [Google Scholar]
- [33].Kim SJ, Kwon OY, Yi CH. Effects of manual lymph drainage on cardiac autonomic tone in healthy subjects. Int J Neurosci 2009;119:1105–17. [DOI] [PubMed] [Google Scholar]
- [34].Kojo I, Pertovaara A. The effects of stimulus area and adaptation temperature on warm and heat pain thresholds in man. Int J Neurosci 1987;32:875–80. [DOI] [PubMed] [Google Scholar]
- [35].Lautenbacher S, Huber C, Schöfer D, Kunz M, Parthum A, Weber PG, Roman C, Griessinger N, Sittl R. Attentional and emotional mechanisms related to pain as predictors of chronic postoperative pain: a comparison with other psychological and physiological predictors. PAIN 2010;151:722–31. [DOI] [PubMed] [Google Scholar]
- [36].Lautenbacher S, Prager M, Rollman GB. Pain additivity, diffuse noxious inhibitory controls, and attention: a functional measurement analysis. Somatosens Mot Res 2007;24:189–201. [DOI] [PubMed] [Google Scholar]
- [37].Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain 1997;13:189–96. [DOI] [PubMed] [Google Scholar]
- [38].Lautenbacher S, Roscher S, Strian F. Inhibitory effects do not depend on the subjective experience of pain during heterotopic noxious conditioning stimulation (HNCS): a contribution to the psychophysics of pain inhibition. Eur J Pain 2002;6:365–74. [DOI] [PubMed] [Google Scholar]
- [39].Leung A, Zhao Y, Shukla S. The effect of acupuncture needle combination on central pain processing-an fMRI study the effect of acupuncture needle combination on central pain processing-an fMRI study. Mol Pain 2014;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leung AY, Kim SJ, Schulteis G, Yaksh T. BMC Complementary and the effect of acupuncture duration on analgesia and peripheral sensory thresholds. BMC Complement Altern Med 2008;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Löfgren M, Norrbrink C. Pain relief in women with fibromyalgia: a cross-over study of superficial warmth stimulation and transcutaneous electrical nerve stimulation. J Rehabil Med 2009;41:557–62. [DOI] [PubMed] [Google Scholar]
- [42].Lorenz J, Bromm B. Event-related potential correlates of interference between cognitive performance and tonic experimental pain. Psychophysiology 1997;34:436–45. [DOI] [PubMed] [Google Scholar]
- [43].Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-larrea L. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. PAIN 2013;154:2563–8. [DOI] [PubMed] [Google Scholar]
- [44].Mancini F, Beaumont A, Hu L, Haggard P, Domenico G, Iannetti D. Touch inhibits subcortical and cortical nociceptive responses. PAIN 2015;156:1936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Marchand S, Arsenault P. Spatial summation for pain perception: interaction of inhibitory and excitatory mechanisms. PAIN 2002;95:201–6. [DOI] [PubMed] [Google Scholar]
- [46].Marks LE. Spatial summation in relation to the dynamics of warmth sensation. Int J Biometeorol 1971;15:106–10. [DOI] [PubMed] [Google Scholar]
- [47].Mason AG, Newton JP, Cadden SW. Modulation of an inhibitory jaw reflex by remote noxious stimulation: effects of spatial conditioning factors. Eur J Oral Sci 2007;115:371–7. [DOI] [PubMed] [Google Scholar]
- [48].McVeigh JG, McGaughey H, Hall M, Kane P. The effectiveness of hydrotherapy in the management of fibromyalgia syndrome: a systematic review. Rheumatol Int 2008;29:119–30. [DOI] [PubMed] [Google Scholar]
- [49].Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–9. [DOI] [PubMed] [Google Scholar]
- [50].Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol 1993;66:254–62. [DOI] [PubMed] [Google Scholar]
- [51].Moont R, Pud D, Sprecher E, Sharvit G, Yarnitsky D. “Pain inhibits pain” mechanisms: is pain modulation simply due to distraction? PAIN 2010;150:113–20. [DOI] [PubMed] [Google Scholar]
- [52].Nahman-Averbuch H, Granovsky Y, Sprecher E, Steiner M, Tzuk-Shina T, Pud D, Yarnitsky D. Associations between autonomic dysfunction and pain in chemotherapy-induced polyneuropathy. Eur J Pain 2014;18:47–55. [DOI] [PubMed] [Google Scholar]
- [53].Nahra H, Plaghki L. Modulation of perception and neurophysiological correlates of brief CO 2 laser stimuli in humans using concurrent large fiber stimulation. Somatosens Mor Res 2003;20:139–47. [DOI] [PubMed] [Google Scholar]
- [54].Pagano RL, Assis DV, Clara JA, Alves AS, Dale CS, Teixeira MJ, Fonoff ET, Britto LR. Transdural motor cortex stimulation reverses neuropathic pain in rats : a profile of neuronal activation. Eur J Pain 2011;15:268.e1–14. [DOI] [PubMed] [Google Scholar]
- [55].Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science 2002;295:1737–40. [DOI] [PubMed] [Google Scholar]
- [56].Platon B, Andréll P, Raner C, Rudolph M, Dvoretsky A, Mannheimer C. High-frequency, high-intensity transcutaneous electrical nerve stimulation as treatment of pain after surgical abortion. PAIN 2010;148:114–9. [DOI] [PubMed] [Google Scholar]
- [57].Price DD, McHaffie JG. Effects of heterotopic conditioning stimuli on first and second pain: a psychophysical evaluation in humans. PAIN 1988;34:245–52. [DOI] [PubMed] [Google Scholar]
- [58].Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. PAIN 2009;144:16–19. [DOI] [PubMed] [Google Scholar]
- [59].Randich A, Maixner W. Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev 1984;8:343–67. [DOI] [PubMed] [Google Scholar]
- [60].Sato D, Yamashiro K, Onishi H, Baba Y, Nakazawa S. Whole-body water flow stimulation to the lower limbs Modulates excitability of primary motor cortical regions Innervating the hands: a transcranial magnetic stimulation study. PLoS One 2014;9:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sato D, Yamashiro K, Onishi H, Yasuhiro B, Shimoyama Y, Maruyama A. Whole-hand water flow stimulation increases motor cortical excitability : a study of transcranial magnetic stimulation and movement-related cortical potentials. J Neurophysiol 2015;113:822–33. [DOI] [PubMed] [Google Scholar]
- [62].Sedan O, Sprecher E, Yarnitsky D. Vagal stomach afferents inhibit somatic pain perception. PAIN 2005;113:354–9. [DOI] [PubMed] [Google Scholar]
- [63].Shukla S, Torossian A, Duann J, Leung A. The analgesic effect of electroacupuncture on acute thermal pain perception-a central neural correlate study with fMRI the analgesic effect of electroacupuncture on acute thermal pain perception-a central neural correlate study with fMRI. Mol Pain 2011;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Staud R, Robinson ME, Goldman CT, Price DD. Attenuation of experimental pain by vibro-tactile stimulation in patients with chronic local or widespread musculoskeletal pain. Eur J Pain 2011;15:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Theysohn N, Choi KE, Gizewski ER, Wen M, Rampp T, Gasser T, Dobos GJ, Forsting M, Musial F. Acupuncture-related modulation of pain-associated brain networks during electrical pain stimulation: a functional magnetic resonance imaging study. J Altern Complement Med 2014;20:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Treede RD. Gain control mechanisms in the nociceptive system. PAIN 2016;157:1199–204. [DOI] [PubMed] [Google Scholar]
- [67].Triedman JK, Saul JP. Blood pressure modulation by central venous pressure and respiration. Buffering effects of the heart rate reflexes. Circulation 1994;89:169–79. [DOI] [PubMed] [Google Scholar]
- [68].Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? PAIN 2002;95:195–9. [DOI] [PubMed] [Google Scholar]
- [69].Wall PD. The gate control theory of pain mechanisms. A re-examination and re-statement. Brain 1978;101:1–18. [DOI] [PubMed] [Google Scholar]
- [70].Wiener A, Mizrahi J, Verbitsky O. Enhancement of tibialis anterior recovery by intermittent sequential pneumatic compression of the legs. Basic Appl Myol 2001;11:87–90. [Google Scholar]
- [71].Willer JC, De Broucker T, Le Bars D. Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. J Neurophysiol 1989;62:1028–38. [DOI] [PubMed] [Google Scholar]
- [72].Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339. [DOI] [PubMed] [Google Scholar]
- [73].Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen KB, Stubhaug A, Treede RD, Wilder-Smith OHG. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19:805–6. [DOI] [PubMed] [Google Scholar]
- [74].Zelikovski A, Ben-Tov I, Koren A, Stelman E, Haddad M. “Veno-Press”–a new sequential intermittent pneumatic device for the prevention of perioperative deep vein thrombosis. Isr J Med Sci 1996;32:1335–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


