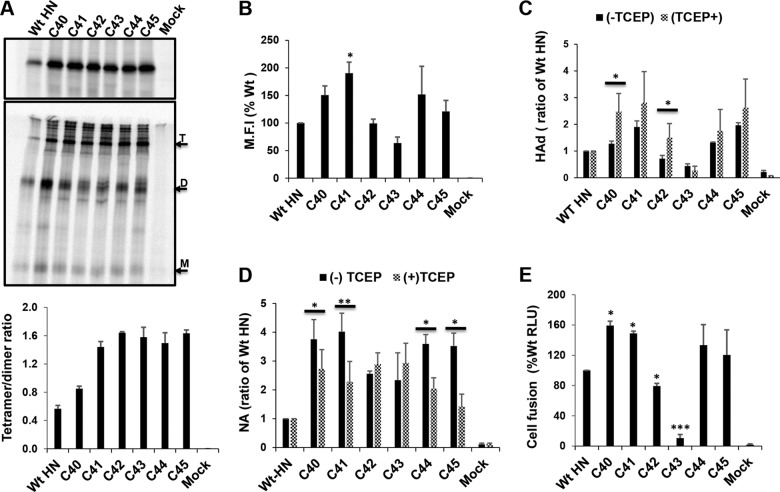
FIG 5.
Disulfide-linked stabilization of HN MPSR. (A) 293T cells expressing HN with cysteine mutations at residues 40 to 45 were 35S metabolically labeled and HN immunoprecipitated, and polypeptides were analyzed by reducing (top) and nonreducing (middle) 10% SDS-PAGE. The different mobilities of WT HN and the mutants are indicated by the monomer (M), dimer (D), and tetramer (T). ImageJ was used to quantify band intensity, and the ratio of tetramer to dimer was estimated (bottom). (B) Cell surface expression of WT HN and cysteine mutants and (C) the effect of disulfide linked tetramerization of the MPSR on receptor binding (HAd) were measured under reducing (10 mM TCEP) and nonreducing conditions. Asterisks indicate statistical significance between TCEP treated and untreated. (D) NA activity of Cys mutants under reducing (TCEP) and nonreducing conditions. (E) Quantitative luciferase fusion assay of WT HN and HN cysteine mutants. *, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05.