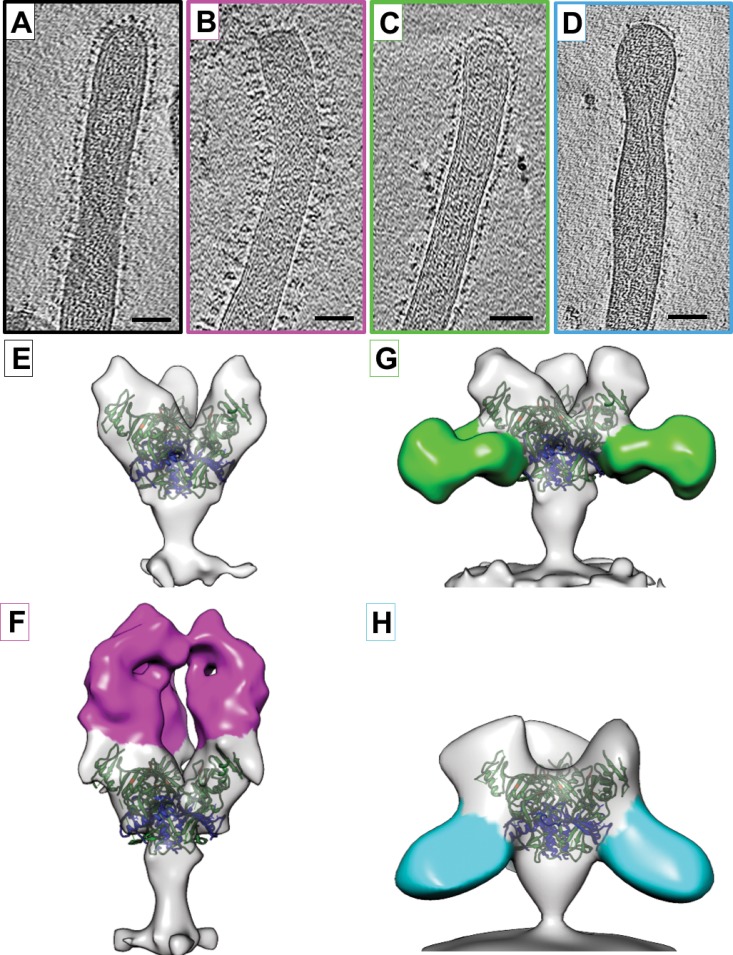
FIG 1.
Structures of EBOV-Makona GP bound to ZMapp antibodies. (A to D) Tomographic slices of EBOV-Makona VLPs are shown unbound (A) or bound to ZMapp antibodies c13C6 (B), c4G7 (C), or c2G4 (D). (E to H) Isosurface representations of density maps derived from tomographic subvolume averaging of glycoprotein spikes expressed on the VLP surface for unbound GP (E) or GP bound to c13C6 (F), c4G7 (G), or c2G4 (H). Density assigned to the antibody Fab region is colored magenta (c13C6), green (c4G7), or cyan (c2G4). All structures have been fitted with the crystal coordinates for EBOV-Mayinga GP (Protein Data Bank [PDB] accession number 3CSY), which lacks the mucin-like domain and the transmembrane domain (21). X-ray coordinates are colored with the GP1 region in green, GP2 in blue, and residues important for binding to NPC1 in orange. Scale bars, 50 nm.