Abstract
Rectal cancer represents about 30% of colorectal cancers, being around 50% locally advanced at presentation. Chemoradiation (CRT) followed by total mesorectal excision is the standard of care for these locally advanced stages. However, it is not free of adverse effects and toxicity and the complete pathologic response rate is between 10% and 30%. This makes it extremely important to define factors that can predict response to this therapy. Focal adhesion kinase (FAK) expression has been correlated with worse prognosis in several tumours and its possible involvement in cancer radio‐ and chemosensitivity has been suggested; however, its role in rectal cancer has not been analysed yet. To analyse the association of FAK expression with tumour response to CRT in locally advanced rectal cancer. This study includes 73 patients with locally advanced rectal cancer receiving standard neoadjuvant CRT followed by total mesorectal excision. Focal adhesion kinase protein levels were immunohistochemically analysed in the pre‐treatment biopsies of these patients and correlated with tumour response to CRT and patients survival. Low FAK expression was significantly correlated with local and distant recurrence (P = 0.013). Low FAK expression was found to be a predictive marker of tumour response to neoadjuvant therapy (P = 0.007) and patients whose tumours did not express FAK showed a strong association with lower disease‐free survival (P = 0.01). Focal adhesion kinase expression predicts neoadjuvant CRT response in rectal cancer patients and it is a clinically relevant risk factor for local and distant recurrence.
Keywords: rectal cancer, chemoradiotherapy, focal adhesion kinase, predictive marker, risk factor, neoadjuvant therapy
Introduction
Rectal cancer represents about 30% of colorectal cancers 1, remaining a significant problem worldwide. In the majority of cases the disease is localized to the primary site with no evidence of distant spread, and in these patients surgical resection currently remains the cornerstone of treatment. Pre‐operative chemoradiation (CRT) and then total mesorectal excision is currently the standard of care for locally advanced stages of rectal cancer, to reduce the probability of recurrence and to possibly improve overall survival 2, 3. The use of neoadjuvant CRT results in a reduction in local recurrence rates when compared to surgery alone 3, 4.
The tumour response to neoadjuvant therapy is assessed as tumour and nodal downstaging and tumour regression grade, which has been correlated with a better outcome 5, 6. While complete response is only observed in 10–30% of cases, in the rest of the cases the residual disease varies from microscopic tumour foci to no response at all 7. Many factors may predict tumour response to CRT 8, 9, 10, 11, 12 but up to now, a model that would predict clinically or pathologically complete or partial tumour response after CRT is not available.
Focal adhesion kinase (FAK) is a cytoplasmic non‐receptor tyrosine kinase that is expressed ubiquitously and specifically localized in focal adhesions 13. This enzyme is involved in the regulation of cell cycle, adhesion, migration and differentiation. The best characterized mechanism that promotes FAK activation involves integrin receptor clustering upon the binding of cells to extracellular matrix proteins. Indirect interactions between FAK and integrins at focal adhesions mediate the integrin‐FAK linkage. In head and neck cancer, the blockade of an integrin‐FAK‐cortactin‐JUN N‐terminal kinase 1 (JNK1) signalling cascade through specific antibodies against β1 integrins renders cells sensitive to radiotherapy and delays xenograft growth 14. It has also been found that overexpression of integrin β1, accompanied by increase in cell adhesion and migration through FAK–AKT signalling, is associated with gefitinib resistance in a non‐small cell lung cancer cell line 15. These results suggest a possible role of FAK in cancer radio‐ and chemosensitivity.
Focal adhesion kinase is a multifunctional regulator of cell signalling within the tumour microenvironment 16, 17, 18, however, some of the functions of FAK in tumorigenesis remain under investigation. Although it has been found that FAK is overexpressed and it correlates with worse prognosis in different tumour types 13, over the last years it has been published that weak expression of FAK is an independent predictor of poor patient outcome in some other tumours 19, 20, 21. The role of FAK in locally advanced rectal cancer with respect to tumour response after CRT or to survival is unclear.
In this study, we performed a single‐centre retrospective analysis of 73 patients treated for locally advanced rectal adenocarcinoma with standard neoadjuvant CRT. Our objective was to investigate FAK expression as a potential predictive marker of preoperative CRT and its correlation with survival.
Materials and methods
Patients and treatment
The records of 91 consecutive patients with clinical American Joint Committee on Cancer 22 stage II or stage III rectal adenocarcinoma who underwent standardized pre‐operative CRT followed by total mesorectal excision, from December 2006 to January 2014, were reviewed. Only those patients with available endoscopic biopsies for immunohistochemical analysis were selected for this study (n = 73). This study was reviewed and approved by the Institutional Review Board at the University Hospital Fundacion Jimenez Diaz and it was performed according to the REMARK guidelines 23.
Magnetic resonance imaging (MRI), computed tomography, endorectal ultrasound and/or endoscopy were used for staging before CRT in all patients. All the cases were reviewed and, according to the recommendations by the College of American Pathologists, a two‐tiered system was used to grade tumours in two groups: low‐grade (greater than or equal to 50% gland formation) and high‐grade (less than 50% gland formation) 24.
Patients received standard pre‐operative radiotherapy with a dose of 40–50.4 Gy in 1.8–2 Gy/fraction. Concomitant fluoropyrimidines‐based chemotherapy (standard regimen of 5‐FU or capecitabine) was administered. In 14 (19%) of the patients, 5‐FU was combined with oxaliplatin. Follow‐up starts at the beginning of the neoadjuvant therapy. Evaluation of T and N stages was determined after CRT, using MRI. The characteristics of the patients and tumours are summarized in Table 1.
Table 1.
Clinicopathological characteristics of the patients
| Baseline clinical characteristics (N = 73) | N (%) |
|---|---|
| Age | |
| <60 | 12 (16.4) |
| >60 | 61 (83.6) |
| Gender | |
| Male | 46 (63.0) |
| Female | 27 (37.0) |
| ECOG Performance status | |
| 0 | 40 (54.8) |
| 1 | 31 (42.5) |
| 2 | 2 (2.7) |
| Neoadjuvant chemotherapy | |
| RT + 5‐FU | 59 (80.8) |
| RT + 5‐FU+oxaliplatin | 14 (19.2) |
| Grade differentiation | |
| Low | 57 (78.1) |
| High | 16 (21.9) |
| Stage | |
| II | 4 (5.5) |
| III | 68 (93.2) |
| N/A | 1 (1.4) |
| Pathological response | |
| Responder | 36 (49.3) |
| Non‐responder | 37 (50.7) |
| T‐Downstaging | |
| No | 28 (38.4) |
| Yes | 39 (53.4) |
| N/A | 6 (8.2) |
| N‐Downstaging | |
| No | 20 (27.4) |
| Yes | 47 (64.4) |
| N/A | 6 (8.2) |
| Status | |
| Death | 8 (11.0) |
| Alive with disease | 6 (8.2) |
| Alive without disease | 58 (79.5) |
| Loss of follow‐up | 1 (1.4) |
ECOG: Eastern Cooperative Oncology Group; RT: radiotherapy; N/A: not available.
Histological grading of tumour regression
To evaluate regression grade, T‐downstaging and N‐downstaging, all the specimens of rectal resection following neoadjuvant therapy were fixed with formaldehyde for 24 hrs. The complete tumour area was embedded in paraffin blocks and histologically analysed. Serial sections were cut from each block and stained with haematoxylin and eosin. Histological assessment of tumour regression was performed by an experienced pathologist. The regression grade was evaluated in the area with the least response to treatment. The percentage of viable residual tumour cells was estimated according to recommendations settled by the College of American Pathologists. Ryan's criteria were used to quantify response as follows: grade 0 (complete response: absence of tumour cells); grade 1 (moderate response: fibrosis with isolated tumour cells); grade 2 (minimal response: tumour nests outgrown by fibrosis); and grade 3 (poor response: minimal or no tumour kill). T‐downstaging was considered to be any reduction in the pathologic T stage versus pre‐treatment stage. N‐downstaging was defined as post‐operative N stage lower than pre‐radiotherapy clinical N stage.
Immunohistochemical evaluation
Formalin‐fixed paraffin‐embedded (FFPE) tissue samples obtained by punch biopsy before pre‐operative CRT were used for Tissue Microarray (TMA) construction. Representative tumour regions from biopsies were identified by a pathologist on haematoxylin and eosin‐stained tissue sections. After pathologist review, TMAs were assembled from triplicate 0.6 mm cores of FFPE biopsy tumour samples using a TMA workstation MTA‐1 (Beecher Instruments, Sun Prairie, WI, USA).
All the immunohistochemical techniques were performed in the Pathology Department at Fundacion Jimenez Diaz. The immunohistochemistry procedure was performed with a Dako Autostainer (Dako, Glostrup, Denmark). The sections were stained using the rabbit polyclonal anti‐FAK (1:50; Cell Signaling, Danvers, MA, USA) which recognizes total FAK protein. Immunohistochemistry was evaluated by two independent pathologists with expertise in gastrointestinal pathology. Optic microscopes were used to evaluate both the percentage of stained cells and also the intensity of staining with a semiquantitative scale in three grades. To asses reliability we measured interobserver agreement with over 90% concordance. Cases with discordant results were reviewed co‐jointly to reach consensus. Both pathologists were blinded to the outcome of the patients.
Statistical analysis
Correlation between FAK expression and clinicopathological variables or response to neoadjuvant therapy was evaluated by Mann–Whitney test. The relationship between FAK expression and each clinical variable with the pathological response was assessed using binary logistic regression model in both univariate and multivariate.
To assess the association of FAK expression with survival, expression versus no expression was used as a cut‐off point. Disease‐free survival was defined as any relapse from the time of surgical procedure. Overall survival was calculated from the time of surgical procedure to the date of death or last follow‐up. Survival analysis was assessed by using the Kaplan–Meier method, with log‐rank test assessing the differences between the groups of FAK expression. Cox regression model was conducted to assess the hazard ratio. Multivariate analysis was performed adjusting for known confounders as age, stage, grade of differentiation and adjuvant treatment. Values of P < 0.05 were considered statistically significant. All reported P‐values were two‐sided. The SPSS software version 20.0 (IBM company, Armonk, NY, USA) was used for all the statistical analyses.
Results
Ninety‐one consecutive rectal cancer patients were reviewed. Endoscopic biopsies before CRT were available for 73 patients (46 men and 27 women) that were finally included in the study. The median age was 74 years (range 48–90). Evaluation of tumour response to pre‐operative CRT showed that 9 patients (12%) had a complete response and 27 (36%) had only isolated tumour cells, all these patients were considered as responders. Patients with minimal and poor response, 15 (21%) and 22 (30%), respectively, were grouped as non‐responders. Thirty‐nine patients (53%) and 47 patients (64%) had T‐ and N‐downstaging, respectively, after CRT. After surgery, 6 of 73 patients developed a local recurrence and 13 patients developed distant metastasis.
Correlation between FAK expression and clinicopathological parameters
Immunohistochemical analysis in tissue samples collected before CRT was performed to determine the FAK expression level. A human bladder tissue was used as a positive control for immunohistochemical staining for FAK (according to the human protein atlas at http://www.proteinatlas.org). Figure 1 shows the cytosolic localization of FAK and results of immunohistochemical staining revealed that median percentage of cells staining positive for FAK was 60 (range 0–90). Eight of 73 patients (11%) were negative for staining and the rest of cases (89%) showed a wide range of FAK expression. There were no significant differences in FAK expression levels with respect to age, gender, ECOG performance status, TNM stage and grade of differentiation (Table 2). However, a significant correlation was found between low FAK expression and the incidence of local and distant metastases (P = 0.013; Fig. 2A, Table 2) since 5 of the 16 patients (31%) with recurrence had no expression of FAK compared to 3 of the 57 patients (5%) without any recurrence.
Figure 1.
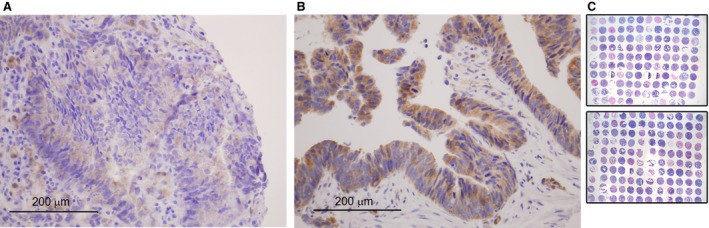
Representative pictures of FAK expression and whole Tissue Microarrays. Immunohistochemical analysis showing weak (A) and strong (B) specific cytoplasmic expression of FAK in rectal cancer biopsies before CRT. (C) Haematoxylin and eosin staining of triplicate cores of all biopsy tumour samples used for the assessment of FAK expression.
Table 2.
Correlation of FAK and clinicopathological parameters
| Variable | Median | Interquartile range | P |
|---|---|---|---|
| Age | |||
| <60 | 65 | 53 | 0.600 |
| >60 | 50 | 45 | |
| Gender | |||
| Male | 55 | 51 | 0.908 |
| Female | 60 | 50 | |
| ECOG performance status | |||
| 0 | 55 | 58 | 0.953 |
| 1 | 60 | 40 | |
| Stage | |||
| II | 30 | 18 | 0.141 |
| III | 60 | 45 | |
| Grade of differentiation | |||
| Low | 60 | 48 | 0.191 |
| High | 35 | 54 | |
| T‐Downstaging | |||
| No | 45 | 39 | 0.169 |
| Yes | 60 | 50 | |
| N‐Downstaging | |||
| No | 45 | 34 | 0.297 |
| Yes | 60 | 50 | |
| Local and distant recurrence | |||
| No | 60 | 50 | 0.013 |
| Yes | 25 | 60 | |
| Pathological response | |||
| No | 40 | 40 | 0.007 |
| Yes | 65 | 44 | |
ECOG: Eastern Cooperative Oncology Group. Bold P values are significant at the 0.05 level.
Figure 2.
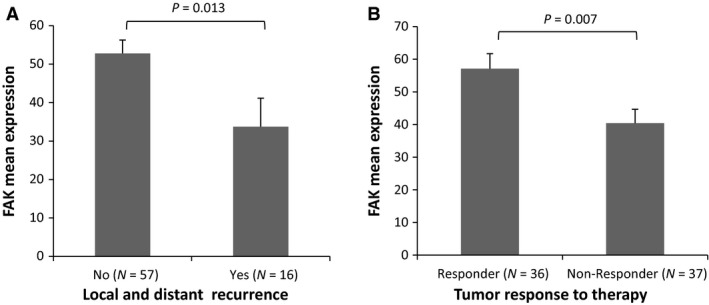
Boxplots show distribution of FAK expression according to local and distant recurrence (A), and tumour response to therapy (B). Significant changes in mean expression for FAK between subgroups were calculated by Mann–Whitney test and indicated in the boxplot.
Tumour response and survival analysis
No significant correlations between FAK protein levels and T‐downstaging or N‐downstaging were observed (Table 2). Nevertheless, FAK expression levels were significantly associated with the histopathological tumour response to CRT, the mean expression was lower in non‐responder patients than responders (P = 0.007; Fig. 2B, Table 2). Binary logistic regression model was performed to assess FAK expression as predictor of response. Univariate analysis showed an association with response (OR: 1.02, 95% CI: 1.00–1.04, P = 0.012), that was maintained as significant after adjustment for potential confounders, age and gender, suggesting FAK as an independent predictor of pathological response.
With a median follow‐up of 32 months (range 7–97 months), 16 of 73 patients developed local recurrence and/or distant metastasis. No expression of FAK was found to be significantly associated with reduced disease‐free survival (P = 0.01 by the log‐rank test, Fig. 3; HR: 3.56; 95% CI: 1.22–10.38, P = 0.02). The 1‐, 3‐ and 5‐year survival rates for these patients were 75%, 45% and 45% respectively. The median survival time was 36 months. Patients whose tumours expressed FAK had 1‐, 3‐ and 5‐year survival rates of 95%, 82% and 82% respectively and the median survival time was not reached. A multivariate analysis was conducted using the confounding variables age, stage, grade of differentiation and adjuvant treatment as covariates. FAK expression remained as an independent prognostic marker of lower risk of recurrence (HR: 4.56; 95% CI: 1.44–14.45, P = 0.010; Table S1). Overall survival was also assessed but it did not meet statistical significance (P = 0.254).
Figure 3.
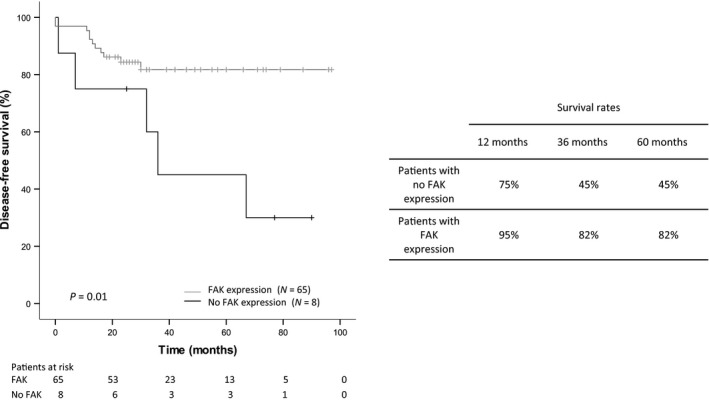
Kaplan–Meier survival analyses of rectal cancer patients receiving neoadjuvant CRT treatment according to FAK expression levels in pre‐treatment biopsies. Patients in the FAK expression group showed significantly better disease‐free survival than those in the no expression group. Data are based on immunohistochemical results of pre‐treatment biopsies.
Discussion
Pre‐operative CRT is the standard of care for patients with locally advanced rectal cancer 2, 3. After this treatment a complete tumour response is observed in 10–30% of cases 7, since all patients do not have the same sensitivity to CRT. Analysis of predictive factors of response to this pre‐operative treatment could help physicians to distinguish between patients that are therefore more likely to respond well from those unlikely to do so.
The role of FAK in radiation sensitivity has been poorly studied and it seems to be controversial. There is a report showing that overexpression of FAK in HL‐60 cells confers marked resistance 25 and another in which siRNA‐mediated FAK knock‐down promotes radio‐sensitization in pancreatic cancer cells 26. However, a study on advanced squamous cancer cells showed that the absence of FAK can release constrained FAK places on signalling from p53 to the induction of several target genes, namely p21 and a subset of genes involved in DNA repair. Moreover, cells without FAK appear to be more efficient at repair after radiation‐induced DNA damage. Thus, FAK functions to suppress the radiation‐induced DNA repair by blocking induction of p21, consequently loss of FAK is linked to enhanced resistance to ionizing radiation 27. Our results are in accordance with this study since we observed that low FAK levels were significantly associated with the absence of response (minimal and poor response) to neoadjuvant CRT treatment supporting that FAK loss induces radio‐resistance in rectal cancer patients and it could be a potential predictive marker. Considering the increasing evidence that the treatment of rectal cancer must be individualized, if this biomarker is validated, it might be used for stratifying patients and included in models for prediction, and therefore adapts the treatment to the patient.
On the other hand, rectal cancer behaves differently from colon cancer, particularly with respect to an increased risk for local recurrence because of the differences in the lymphatic drainage and the narrow anatomic space of the pelvis. Although the reduction in the rate of local recurrence is higher after pre‐operative radiotherapy, when compared to post‐operative radiation alone in primary local advanced rectal cancer cases, about 20% patients will suffer recurrence or distant metastasis 28. It has been found that risk factors associated with local recurrence in patients with rectal cancer receiving neoadjuvant therapy are different from the traditional factors in patients treated with surgery and/or adjuvant therapy alone 29.
Focal adhesion kinase is typically located at structures known as focal adhesions from where it transduces signals into cells that control multiple cancer‐related events, including migration, invasion, angiogenesis, protection of cells from suspension‐induced cell death and proliferation in three‐dimensional matrices 30, 31, 32, 33, 34.
In this study, we observed that no FAK expression was significantly associated with a shorter DFS, suggesting FAK as a potential risk factor. This result would be supported by molecular studies which describe an increased carcinoma cell migration after inhibition of FAK in HeLa cervical cancer cells 35 and a negative role for FAK during the invasion of different types of carcinoma cells 36. These studies show that the down‐regulation of FAK is required and sufficient for detachment of cells from the extracellular matrix, leading to increased tumour cell motility, invasion and metastasis. The detached cells migrate to a new site, followed by reactivation of FAK and reattachment to the extracellular matrix, developing metastatic deposits. It is also supported by experiments using FAK knockdown cells which exhibited remarkably enhanced motility after detachment and migrated out from the cells sheet in wound‐healing assay. The down‐regulation of FAK is essential for early metastatic spreading, enabling vascular circulation of tumour cells without adhesion. Following this, rectal cancer patients with no FAK expression in the biopsy before pre‐operative CRT are more likely to have detached cells responsible for future metastases than those patients expressing FAK. It is expected that these patients have higher incidence of recurrence and worse disease‐free survival as we observed in our work.
This is the first report describing the impact of FAK expression in rectal cancer patients as a predictive and prognostic marker. The association of low FAK levels with the outcome in these patients is an unexpected finding since most of the previous studies in different tumour types point high levels of FAK as a prognostic biomarker and potential therapeutic target 13. However, recent studies have described that weak expression of FAK is a strong independent predictor of poorer disease outcome. In human cervical cancer, patients with low expression of FAK were characterized by a significantly poor overall survival 20. Interestingly, these authors also found a significant inverse correlation between FAK expression and pelvic lymph node metastasis, recurrent disease and survival status. These observations are in accordance to our results in rectal cancer. Reduced expression of FAK has also been associated with poor survival in intrahepatic cholangiocarcinoma 37, extrahepatic bile duct carcinoma 38 and ovarian cancer 39. The association of low FAK levels and outcome has also been observed in haematological malignancies. Focal adhesion kinase expression was an independent prognostic factor and showed a tendency to be associated with tumour response to therapy in diffuse large B‐cell lymphoma 19. In chronic lymphocytic leukaemia, increased FAK expression was associated with improved outcome in patients treated with rituximab, fludarabine and cyclophosphamide immunochemotherapy 21. All together suggests that FAK might play different roles in different tumour types or during different stages of tumour progression.
So far, most of the studies have suggested that FAK functions drive various tumour‐promoting signalling pathways, and small‐molecule FAK inhibitors are emerging as promising chemotherapeutics which are being evaluated in different clinical trials 13. However, it is important to record that FAK's role in cellular responses to ionizing radiation, and pro‐survival signalling in general, may be context dependent, and that there needs to be caution when considering therapeutic combinations of FAK inhibitors and radiotherapy, as this may not always be clinically beneficial.
One of the limitations to our study that warrant consideration is that it has been performed in a limited cohort. To address whether FAK expression could be a suitable marker for identifying patients at higher risk in rectal cancer, we are continuing to collect specimens from additional patients to validate our results as well as extending our studies to an independent patient cohort.
In conclusion, we have identified low FAK expression associated with tumour resistance to neoadjuvant CRT and worse disease‐free survival in rectal cancer patients. This is an important finding because it helps to identify a subset of patients with rectal cancer who will most likely not respond to CRT.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Author contribution
TGP and AC designed the study, analysed the data and wrote the paper; MJFA interpreted the immunohistochemical results and analysed the data; ABP, JMU, RVB and MRR performed the research; LPN generated the clinical database and analysed the data; JPMA, CC, and MCR reviewed the records of rectal cancer patients; FM interpreted the Immunohistochemical results; JGF designed the study and revised the paper critically.
Supporting information
Table S1 Univariate and multivariate analyses of FAK expression and disease‐free survival in rectal cancer patients.
Acknowledgements
The authors acknowledge the role of the Fundacion Jimenez Diaz Hospital Biobank in collecting and making available the samples used in order to conduct this publication. This work was supported by the Consolider Ingenio Program from the Spanish Ministry of Economy and Competitiveness (Consolider‐Ingenio RNAREG CSD2009‐00080).
References
- 1. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014; 64: 104–17. [DOI] [PubMed] [Google Scholar]
- 2. Madoff RD. Rectal cancer: optimum treatment leads to optimum results. Lancet. 2009; 373: 790–2. [DOI] [PubMed] [Google Scholar]
- 3. Sauer R, Becker H, Hohenberger W, et al Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351: 1731–40. [DOI] [PubMed] [Google Scholar]
- 4. Swedish Rectal Cancer Trial . Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997; 336: 980–7. [DOI] [PubMed] [Google Scholar]
- 5. Kaminsky‐Forrett MC, Conroy T, Luporsi E, et al Prognostic implications of downstaging following preoperative radiation therapy for operable T3‐T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998; 42: 935–41. [DOI] [PubMed] [Google Scholar]
- 6. Vecchio FM, Valentini V, Minsky BD, et al The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005; 62: 752–60. [DOI] [PubMed] [Google Scholar]
- 7. Wheeler JM, Dodds E, Warren BF, et al Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: correlation with rectal cancer regression grade. Dis Colon Rectum. 2004; 47: 2025–31. [DOI] [PubMed] [Google Scholar]
- 8. Das P, Skibber JM, Rodriguez‐Bigas MA, et al Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007; 109: 1750–5. [DOI] [PubMed] [Google Scholar]
- 9. Park HC, Janjan NA, Mendoza TR, et al Temporal patterns of fatigue predict pathologic response in patients treated with preoperative chemoradiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2009; 75: 775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park YA, Sohn SK, Seong J, et al Serum CEA as a predictor for the response to preoperative chemoradiation in rectal cancer. J Surg Oncol. 2006; 93: 145–50. [DOI] [PubMed] [Google Scholar]
- 11. Theodoropoulos G, Wise WE, Padmanabhan A, et al T‐level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease‐free survival. Dis Colon Rectum. 2002; 45: 895–903. [DOI] [PubMed] [Google Scholar]
- 12. Yoon SM, Kim DY, Kim TH, et al Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007; 69: 1167–72. [DOI] [PubMed] [Google Scholar]
- 13. Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014; 14: 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eke I, Deuse Y, Hehlgans S, et al beta(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest. 2012; 122: 1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ju L, Zhou C. Integrin beta 1 enhances the epithelial‐mesenchymal transition in association with gefitinib resistance of non‐small cell lung cancer. Cancer Biomark. 2013; 13: 329–36. [DOI] [PubMed] [Google Scholar]
- 16. Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003; 116: 1409–16. [DOI] [PubMed] [Google Scholar]
- 17. Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010; 123: 1007–13. [DOI] [PubMed] [Google Scholar]
- 18. Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009; 28: 35–49. [DOI] [PubMed] [Google Scholar]
- 19. Bosch R, Dieguez‐Gonzalez R, Moreno MJ, et al Focal adhesion protein expression in human diffuse large B‐cell lymphoma. Histopathology. 2014; 65: 119–31. [DOI] [PubMed] [Google Scholar]
- 20. Gabriel B, zur Hausen A, Stickeler E, et al Weak expression of focal adhesion kinase (pp125FAK) in patients with cervical cancer is associated with poor disease outcome. Clin Cancer Res. 2006; 12: 2476–83. [DOI] [PubMed] [Google Scholar]
- 21. Weisser M, Yeh RF, Duchateau‐Nguyen G, et al PTK2 expression and immunochemotherapy outcome in chronic lymphocytic leukemia. Blood. 2014; 124: 420–5. [DOI] [PubMed] [Google Scholar]
- 22. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17: 1471–4. [DOI] [PubMed] [Google Scholar]
- 23. McShane LM, Altman DG, Sauerbrei W, et al REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur J Cancer. 2005; 41: 1690–6. [DOI] [PubMed] [Google Scholar]
- 24. Compton CC, Fielding LP, Burgart LJ, et al Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000; 124: 979–94. [DOI] [PubMed] [Google Scholar]
- 25. Kasahara T, Koguchi E, Funakoshi M, et al Antiapoptotic action of focal adhesion kinase (FAK) against ionizing radiation. Antioxid Redox Signal. 2002; 4: 491–9. [DOI] [PubMed] [Google Scholar]
- 26. Cordes N, Frick S, Brunner TB, et al Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin‐1. Oncogene. 2007; 26: 6851–62. [DOI] [PubMed] [Google Scholar]
- 27. Graham K, Moran‐Jones K, Sansom OJ, et al FAK deletion promotes p53‐mediated induction of p21, DNA‐damage responses and radio‐resistance in advanced squamous cancer cells. PLoS ONE. 2011; 6: e27806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai Y, Li Z, Gu X, et al Prognostic factors associated with locally recurrent rectal cancer following primary surgery (Review). Oncol Lett. 2014; 7: 10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng JY, Li ZN, Wang Y. Risk factors for local recurrence following neoadjuvant chemoradiotherapy for rectal cancers. World J Gastroenterol. 2013; 19: 5227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sieg DJ, Hauck CR, Ilic D, et al FAK integrates growth‐factor and integrin signals to promote cell migration. Nat Cell Biol. 2000; 2: 249–56. [DOI] [PubMed] [Google Scholar]
- 31. Hauck CR, Sieg DJ, Hsia DA, et al Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor‐stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 2001; 61: 7079–90. [PubMed] [Google Scholar]
- 32. Duxbury MS, Ito H, Zinner MJ, et al Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery. 2004; 135: 555–62. [DOI] [PubMed] [Google Scholar]
- 33. Gao N, He H, Xiao L, et al The effects of focal adhesion kinase on the motility, proliferation and apoptosis of Caco2 and SMMC‐7721 cells. Med Oncol. 2015; 32: 125. [DOI] [PubMed] [Google Scholar]
- 34. Pelillo C, Bergamo A, Mollica H, et al Colorectal cancer metastases settle in the hepatic microenvironment through alpha5beta1 integrin. J Cell Biochem. 2015; 116: 2385–96. [DOI] [PubMed] [Google Scholar]
- 35. Yano H, Mazaki Y, Kurokawa K, et al Roles played by a subset of integrin signaling molecules in cadherin‐based cell‐cell adhesion. J Cell Biol. 2004; 166: 283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu Z, Jiang G, Blume‐Jensen P, et al Epidermal growth factor‐induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001; 21: 4016–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohta R, Yamashita Y, Taketomi A, et al Reduced expression of focal adhesion kinase in intrahepatic cholangiocarcinoma is associated with poor tumor differentiation. Oncology. 2006; 71: 417–22. [DOI] [PubMed] [Google Scholar]
- 38. Hayashi A, Aishima S, Inoue T, et al Decreased expression of focal adhesion kinase is associated with a poor prognosis in extrahepatic bile duct carcinoma. Hum Pathol. 2010; 41: 859–66. [DOI] [PubMed] [Google Scholar]
- 39. Aust S, Auer K, Bachmayr‐Heyda A, et al Ambivalent role of pFAK‐Y397 in serous ovarian cancer–a study of the OVCAD consortium. Mol Cancer. 2014; 13: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Univariate and multivariate analyses of FAK expression and disease‐free survival in rectal cancer patients.


