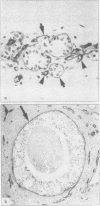
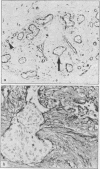
Abstract
The effects of different fixatives and enzymatic digestion procedures on the immunohistochemical demonstration of fibronectin and laminin in paraffin embedded tissues have been compared. None of the fixatives tested enabled staining of these proteins without enzymatic digestion. No intracytoplasmic laminin was found either in fixed or in fresh frozen tissue. Fixation in formol acetic acid was unsatisfactory for demonstration of fibronectin; prolonged fixation in formol sublimate was unsatisfactory for demonstration of laminin. Optimal results were achieved after fixation in routine 10% formol saline. Trypsin was completely ineffective for unmasking laminin antigens except after fixation in ethanol acetic acid; it was only partially effective for showing fibronectin antigens. The best results were obtained with protease digestion, but pepsin was an adequate, although slightly less reliable, alternative. These enzymes may be used at lower concentrations than usually recommended.
Full text
PDF


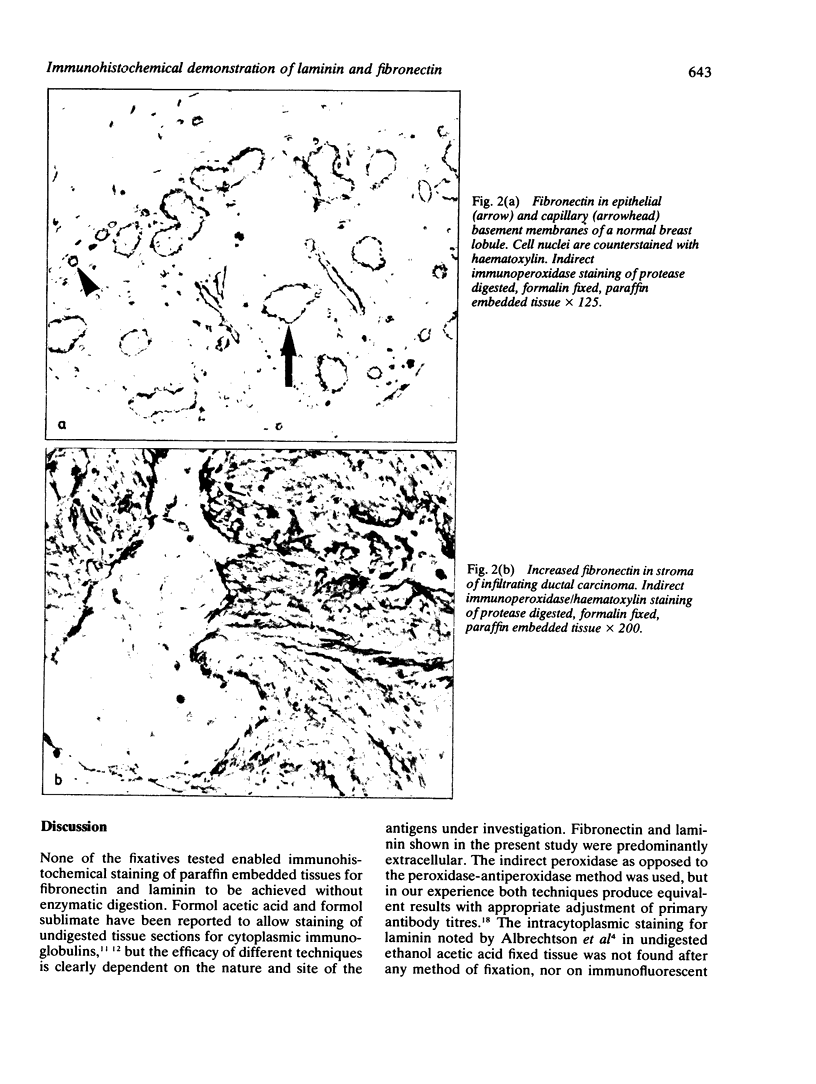

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen R., Nielsen M., Wewer U., Engvall E., Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981 Dec;41(12 Pt 1):5076–5081. [PubMed] [Google Scholar]
- Bosman F. T., Lindeman J., Kuiper G., van der Wal A., Kreunig J. The influence of fixation on immunoperoxidase staining of plasmacells in paraffin sections of intestinal biopsy specimens. Histochemistry. 1977 Jul 18;53(1):57–62. doi: 10.1007/BF00511210. [DOI] [PubMed] [Google Scholar]
- Burns J., Dixon A. J., Woods J. C. Immunoperoxidase localisation of fibronectin in glomeruli of formalin fixed paraffin processed renal tissue. Histochemistry. 1980;67(1):73–78. doi: 10.1007/BF00490089. [DOI] [PubMed] [Google Scholar]
- Curran R. C., Gregory J. Effects of fixation and processing on immunohistochemical demonstration of immunoglobulin in paraffin sections of tonsil and bone marrow. J Clin Pathol. 1980 Nov;33(11):1047–1057. doi: 10.1136/jcp.33.11.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. C., Gregory J. The unmasking of antigens in paraffin sections of tissue by trypsin. Experientia. 1977 Oct 15;33(10):1400–1401. doi: 10.1007/BF01920206. [DOI] [PubMed] [Google Scholar]
- D'Ardenne A. J., Burns J., Sykes B. C., Bennett M. K. Fibronectin and type III collagen in epithelial neoplasms of gastrointestinal tract and salivary gland. J Clin Pathol. 1983 Jul;36(7):756–763. doi: 10.1136/jcp.36.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne A. J., Burns J., Sykes B. C., Kirkpatrick P. Comparative distribution of fibronectin and type III collagen in normal human tissues. J Pathol. 1983 Sep;141(1):55–69. doi: 10.1002/path.1711410107. [DOI] [PubMed] [Google Scholar]
- Du Boulay C. E. Demonstration of fibronectin in soft tissue tumours using the immunoperoxidase technique. Diagn Histopathol. 1982 Oct-Dec;5(4):283–289. [PubMed] [Google Scholar]
- Ekblom P., Miettinen M., Rapola J., Foidart J. M. Demonstration of laminin, a basement membrane glycoprotein, in routinely processed formalin-fixed human tissues. Histochemistry. 1982;75(3):301–307. doi: 10.1007/BF00496733. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Hølund B., Clausen P. P., Clemmensen I. The influence of fixation and tissue preparation on the immunohistochemical demonstration of fibronectin in human tissue. Histochemistry. 1981;72(2):291–299. doi: 10.1007/BF00517142. [DOI] [PubMed] [Google Scholar]
- Mepham B. L., Frater W., Mitchell B. S. The use of proteolytic enzymes to improve immunoglobulin staining by the PAP technique. Histochem J. 1979 May;11(3):345–357. doi: 10.1007/BF01005033. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Foidart J. M., Ekblom P. Immunohistochemical demonstration of laminin, the major glycoprotein of basement membranes, as an aid in the diagnosis of soft tissue tumors. Am J Clin Pathol. 1983 Mar;79(3):306–311. doi: 10.1093/ajcp/79.3.306. [DOI] [PubMed] [Google Scholar]
- Sinclair R. A., Burns J., Dunnill M. S. Immunoperoxidase staining of formalin-fixed, paraffin-embedded, human renal biopsies with a comparison of the peroxidase-antiperoxidase (PAP) and indirect methods. J Clin Pathol. 1981 Aug;34(8):859–865. doi: 10.1136/jcp.34.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendröi M., Labat-Robert J., Godeau G., Robert A. M. Immunohistochemical detection of fibronectin using different fixatives in paraffin embedded sections. Pathol Biol (Paris) 1983 Sep;31(7):631–636. [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]