Abstract
Based on morphology and DNA sequence data the taxonomic relationships of Microdochium, Monographella and Idriella were reassessed. Microdochium is morphologically and phylogenetically circumscribed, and the sexual genus Monographella treated as synonym on the basis that Microdochium has more species, is more commonly encountered, and more frequently used in literature. An epitype is designated for Microdochium phragmites, and several well-known species are redefined based on their morphology and DNA sequence data (LSU, ITS, BTUB and RPB2). Furthermore, the revision of Microdochium led to six new combinations (M. albescens, M. consociatum, M. fusariisporum, M. maydis, M. opuntiae and M. stevensonii) and six new species (M. citrinidiscum, M. colombiense, M. fisheri, M. neoqueenslandicum, M. seminicola and M. trichocladiopsis) being proposed. Microdochium s.str. belongs to a monophyletic clade, together with Idriella lunata and Selenodriella, representing a new family, Microdochiaceae, in Xylariales. Other species previously accommodated in Microdochium belong to different orders in the Ascomycota. Microdochium gracile belongs to Sordariomycetes (incertae sedis) and Paramicrodochium is proposed to accommodate this species. Microdochium tripsaci belongs to Ephelis in Clavicipitaceae, while M. fusarioides belongs to a new genus, Microdochiella in Orbiliales. Idriella s.str. is a monotypic genus phylogenetically closely related to Microdochium. Idriella s.l. separates into different genera in Xylariales (incertae sedis) including Castanediella, Selenodriella, Idriellopsis, Neoidriella and Paraidriella, the last three proposed here as new genera.
Keywords: cereals, grasses, phytopathogenic fungi, Sordariomycetes, Xylariales
INTRODUCTION
Microdochium was introduced with M. phragmitis as the type species for a fungus observed on living leaves of Phragmites australis in Germany, with globose, erumpent stromata of minute, hyaline cells, small papillate conoid conidiogenous cells and solitary, fusiform to subfalcate, hyaline conidia (Sydow 1924). Currently this genus includes about 20 species (Seifert et al. 2011), but only a few of them are well-known and have been studied in pure culture. Braun (1995) recognised three sections in Microdochium based on the type of conidiogenous cells and conidia: Microdochium sect. Gerlachia for species with annellidic conidiogenous cells with percurrent proliferations; Microdochium sect. Microdochium for species with sympodial, often subdenticulate conidiogenous cells, and fairly more or less fusiform, straight to somewhat curved or falcate, 0–3-septate or even pluriseptate conidia; and Microdochium sect. Gloeocercospora for species with sympodial conidiogenous cells, and very long, scolecosporous and pluriseptate conidia. The sexual morphs of Microdochium species are known to reside in Monographella (Amphisphaeriaceae, Xylariales) (Parkinson et al. 1981, Samuels & Hallet 1983, Von Arx 1984, Jaklitsch & Voglmayr 2012). Monographella species are characterised by the production of perithecia immersed in leaf sheaths in natural substrates. In culture perithecia are superficial, globose, with clavate periphyses, show a peridium composed by isodiametric to subglobose cells of textura angularis-epidermoidea, and apically free paraphyses. Asci are oblong to clavate, with eight biseriate ascospores, and with a refractive, amyloid, flat, funnel-shaped apical ring. Ascospores are fusiform or oblong, hyaline, straight or slightly curved, and smooth. Monographella presently includes 11 species.
Microdochium and Monographella include important plant pathogens, particularly on grasses and cereals. In cold to temperate regions ‘Microdochium patch’, also known as ‘pink snow mould’ or ‘Fusarium patch’, is an economically important disease of wheat and barley, caused by Microdochium nivale (previously M. nivale var. nivale) and M. majus (previously M. nivale var. majus) (Von Arx 1987, Glynn et al. 2005, Jewell & Hsiang 2013). ‘Leaf-scald disease’ of rice is caused by Monographella albescens (Von Arx 1987). Leaf scald has the potential to significantly reduce rice yields through the destruction of leaf surface area, the production of sterile/deformed flowers, and seed decay. Monographella albescens has a worldwide distribution, causing considerable yield losses in India, Latin America and West Africa. In Mexico, Monographella maydis on Zea mays produces a tar-spot disease complex of maize together with Phyllachora maydis (Müller & Samuels 1984, Von Arx 1987, Hock et al. 1992). Microdochium bolleyi is known to produce root necrosis and decay of grasses (Braun 1995, Hong et al. 2008). Monographella opuntiae causes the brown spotting on Opuntia (Von Arx 1987, Braun 1995). Microdochium tripsaci is responsible for a systematic infection on Tripsacum laxum (Von Arx 1987, Braun 1995), while M. sorghi causes zonate leaf spots and decay on Sorghum species and other Poaceae (Von Arx 1987, Braun 1995). Finally, M. paspali is known to produce seashore paspalum disease of Paspalum vaginatum (Zhang et al. 2015).
Microdochium species are recognised as fusarium-like fungi. Nevertheless, the conidiogenous cells in Microdochium spp. are not phialidic as in true Fusarium species and the conidia have a truncate base rather than ‘foot-cells’. Besides, the sexual morphs of Microdochium are known to reside in Monographella. On the other hand, the close affinity of Microdochium to Idriella has been discussed by various authors (Sutton et al. 1972, Mouchacca & Samson 1973, Von Arx 1981). Idriella lunata, the type species of Idriella, which was described as a fungus causing a root rot of strawberry in California, differs in producing dark grey to blackish brown colonies, pale brown conidiophores reduced to conidiogenous cells and short-stalked or sessile, brown chlamydospores (Nelson & Wilhelm 1956). Microdochium and Idriella are very similar genera that have polyblastic conidiogenous cells and hyaline falcate conidia, with the presence of chlamydospores in culture. Von Arx (1981) differentiated both genera based on their habitat and conidial shape. He accommodated saprobic species with falcate or lunate conidia, dark colonies and chlamydospores in Idriella, and retained the phytopathogenic species in Microdochium. Nevertheless, morphological and ecological delimitation of Microdochium and Idriella is problematic and remains obscure, and taxonomic affinities inferred from molecular data have not yet been established. Idriella has been linked to Hymenoscyphus caudatus in the Helotiales (Kimbrough & Atkinson 1972). Currently the genus Idriella includes approximately 30 species (Seifert et al. 2011), but few cultures and ex-type strains are available for comparison.
A number of isolates of Microdochium have accumulated over the years in the culture collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands and in the National Mycological Herbarium from Canada (DAOM), which were formerly identified based on morphology only. The aims of this study were: 1) to characterise these diverse isolates incorporating culture characteristics (macro- and micro-morphology) and molecular data; 2) to delimit the species in Microdochium, Monographella and Idriella based on phylogenetic analysis of multi-gene sequence data and morphological characters; and 3) to resolve taxonomic and nomenclatural uncertainty by providing modern descriptions and designating an epitype for the type species of Microdochium.
MATERIALS AND METHODS
Isolates
Isolates used in this study were obtained from CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands, which included all available ex-type strains of described species. Additional isolates were obtained from the National Mycological Herbarium from Canada (DAOM) (Table 1). Isolates were cultured on oatmeal agar (OA; Crous et al. 2009b), and incubated at 25 °C under daylight conditions for 3 wk. Reference strains were deposited in the CBS culture collection. Taxonomic information and nomenclature for new species were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
Table 1.
Specimens and GenBank accession numbers of DNA sequences used in this study. T = ex-type; ET = ex-epitype.
| Species | Voucher | Host/Substrate | Country | GenBank accession numbers |
|||
|---|---|---|---|---|---|---|---|
| LSU | ITS | BTUB | RPB2 | ||||
| Castanediella cagnizarii | CBS 542.96 T | Leaf litter | Cuba | KP858991 | KP859054 | – | – |
| CBS 101043 | Leaf litter | Brazil | KP858988 | KP859051 | – | – | |
| Castanediella couratarii | CBS 579.71 T | Wood | Brazil | KP858987 | KP859050 | – | – |
| Ephelis tripsaci | CBS 857.72 T | Leaf sheath of Tripsacum laxum | Sri Lanka | KP858978 | KP859042 | – | – |
| Idriella lunata | CBS 204.56 T | Root of Fragaria chiloensis | USA | KP858981 | KP859044 | – | – |
| CBS 177.57 | Unknown | USA | KP858980 | KP859043 | – | – | |
| CBS 209.60 | Soil | The Netherlands | KP858982 | KP859045 | – | – | |
| CBS 736.74 | Unknown | Japan | KP858983 | KP859046 | – | – | |
| Idriellopsis uncinospora | CBS 575.92 T | Decaying leaves | Cuba | KP858989 | KP859052 | – | – |
| Microdochiella fusarioidea | CBS 740.83 | On oospores of Phytophthora syringae | UK | KP858976 | KP859040 | – | – |
| CBS 741.83T | On oospores of Phytophthora syringae | UK | KP858975 | KP859039 | – | – | |
| CBS 742.83 | On oospores of Phytophthora syringae | UK | KP858977 | KP859041 | – | – | |
| Microdochium albescens | CBS 290.79 | On Oryza sativa | Ivory Coast | KP858950 | KP859014 | KP859077 | KP859123 |
| CBS 291.79 | On Oryza sativa | Ivory Coast | KP858932 | KP858996 | KP859059 | KP859105 | |
| CBS 243.83 | Seed Oryza sativa | Unknown country | KP858930 | KP858994 | KP859057 | KP859103 | |
| Microdochium bolleyi | CBS 540.92 | Root of Hordeum vulgare | Syria | KP858946 | KP859010 | KP859073 | KP859119 |
| CPC 25994 | Wood in Rideau River | Canada | KP858954 | KP859018 | KP859081 | KP859127 | |
| Microdochium citrinidiscum | CBS 109067 T | Leaf of Eichhornia crassipes | Peru | KP858939 | KP859003 | KP859066 | KP859112 |
| Microdochium colombiense | CBS 624.94 T | On Musa sapientum | Colombia | KP858935 | KP858999 | KP859062 | KP859108 |
| Microdochium fisheri | CBS 242.91 T | Stem of Oryza sativa | UK | KP858951 | KP859015 | KP859078 | KP859124 |
| Microdochium lycopodinum | CBS 146.68 | Air sample | The Netherlands | KP858929 | KP858993 | KP859056 | KP859102 |
| CBS 109397 | On Phragmites australis | Germany | KP858940 | KP859004 | KP859067 | KP859113 | |
| CBS 109398 | On Phragmites australis | Germany | KP858941 | KP859005 | KP859068 | KP859114 | |
| CBS 109399 | On Phragmites australis | Germany | KP858942 | KP859006 | KP859069 | KP859115 | |
| CBS 122885 T | Leaves of Lycopodium annotinum | Austria | KP858952 | KP859016 | KP859079 | KP859125 | |
| Microdochium majus | CBS 741.79 | On Triticum aestivum | Germany | KP858937 | KP859001 | KP859064 | KP859110 |
| Microdochium neoqueenslandicum | CBS 445.95 | On Juncus effusus | The Netherlands | KP858933 | KP858997 | KP859060 | KP859106 |
| CBS 108926 T | On Agrostis sp. | New Zealand | KP858938 | KP859002 | KP859065 | KP859111 | |
| Microdochium nivale | CBS 116205 T | Roots Triticum aestivum | UK | KP858944 | KP859008 | KP859071 | KP859117 |
| Microdochium phragmitis | CBS 285.71 ET | On Phragmites australis | Poland | KP858949 | KP859013 | KP859076 | KP859122 |
| CBS 423.78 | On Phragmites communis | Germany | KP858948 | KP859012 | KP859075 | KP859121 | |
| Microdochium seminicola | CBS 122706 | Maize kernels | Switzerland | KP858943 | KP859007 | KP859070 | KP859116 |
| CBS 122707 | Maize kernels | Switzerland | KP858947 | KP859011 | KP859074 | KP859120 | |
| CBS 139951 T | Maize kernels | Switzerland | KP858974 | KP859038 | KP859101 | KP859147 | |
| CPC 25993 | On Triticum aestivum | Canada | KP858953 | KP859017 | KP859080 | KP859126 | |
| CPC 26001 | On grain | Canada | KP858961 | KP859025 | KP859088 | KP859134 | |
| CPC 26010 | Unknown | Canada | KP858969 | KP859033 | KP859096 | KP859142 | |
| DAOM 250155 | Maize kernels | Switzerland | KP858973 | KP859037 | KP859100 | KP859146 | |
| DAOM 250158 | Maize kernels | Switzerland | KP858972 | KP859036 | KP859099 | KP859145 | |
| DAOM 250159 | Maize kernels | Switzerland | KP858971 | KP859035 | KP859098 | KP859144 | |
| DAOM 250161 | On Triticum aestivum | Canada | KP858970 | KP859034 | KP859097 | KP859143 | |
| DAOM 250162 | On Triticum aestivum | Canada | KP858968 | KP859032 | KP859095 | KP859141 | |
| DAOM 250163 | Unknown | Canada | KP858967 | KP859031 | KP859094 | KP859140 | |
| DAOM 250165 | On grain | Canada | KP858966 | KP859030 | KP859093 | KP859139 | |
| DAOM 250166 | On grain | Canada | KP858965 | KP859029 | KP859092 | KP859138 | |
| DAOM 250167 | On grain | Canada | KP858964 | KP859028 | KP859091 | KP859137 | |
| DAOM 250168 | On grain | Canada | KP858963 | KP859027 | KP859090 | KP859136 | |
| DAOM 250169 | On grain | Canada | KP858962 | KP859026 | KP859089 | KP859135 | |
| DAOM 250171 | On grain | Canada | KP858960 | KP859024 | KP859087 | KP859133 | |
| DAOM 250172 | On grain | Canada | KP858959 | KP859023 | KP859086 | KP859132 | |
| DAOM 250173 | On grain | Canada | KP858958 | KP859022 | KP859085 | KP859131 | |
| DAOM 250174 | On grain | Canada | KP858957 | KP859021 | KP859084 | KP859130 | |
| DAOM 250175 | On grain | Canada | KP858956 | KP859020 | KP859083 | KP859129 | |
| DAOM 250176 | On Triticum aestivum | Canada | KP858955 | KP859019 | KP859082 | KP859128 | |
| Microdochium sorghi | CBS 691.96 | Living Sorghum halepense | Cuba | KP858936 | KP859000 | KP859063 | KP859109 |
| Microdochium tainanense | CBS 269.76 T | Root of Saccharum officinarum | Taiwan | KP858945 | KP859009 | KP859072 | KP859118 |
| CBS 270.76 | Root of Saccharum officinarum | Taiwan | KP858931 | KP858995 | KP859058 | KP859104 | |
| Microdochium trichocladiopsis | CBS 623.77 T | Rhizosphere of Triticum aestivum | Unknown country | KP858934 | KP858998 | KP859061 | KP859107 |
| Neoidriella desertorum | CBS 985.72 T | Soil | Egypt | KP858985 | KP859048 | – | – |
| Paraidriella jambosae | CBS 374.90 T | Leaves of Syzygium jambos | Cuba | KP858986 | KP859049 | – | – |
| Paramicrodochium gracile | CBS 493.70 T | Rabbit dung | The Netherlands | KP858979 | – | – | – |
| Selenodriella cubensis | CBS 683.96 | Unknown | Cuba | KP858990 | KP859053 | – | – |
| Selenodriella fertilis | CBS 772.83 | Dead leaf of Hakea baxteri | Australia | KP858992 | KP859055 | – | – |
DNA isolation, amplification and analyses
Genomic DNA was extracted from fungal colonies growing on 2 % malt extract agar (MEA; Oxoid) using the UltraClean™ Microbial DNA Isolation kit (MoBio Laboratories, Inc., Solana Beach, CA, USA) and Wizard® Genomic DNA purification kit (Promega, Madison, USA), according to the manufacturer’s protocols. The primers V9G (De Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part (ITS) of the nuclear rDNA operon spanning the 3’ end of the 18S rRNA gene, the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region (ITS2) and ± 900 bp of the 5’ end of the 28S rRNA gene. The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009a) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. Part of the beta-tubulin gene region (BTUB) was amplified and sequenced using primers Btub526F and Btub1332R (Jewell & Hsiang 2013), and primers RPB150F (Jewell & Hsiang 2013) and fRPB2-7cR (Liu et al. 1999) were used for the RNA polymerase II second largest subunit gene (RPB2). Amplification conditions for ITS and LSU followed Crous et al. (2013) and for BTUB and RPB2 Jewell & Hsiang (2013). The program SeqMan Pro (DNASTAR, Madison, WI, USA) was used to obtain consensus sequences of each isolate. Megablast searches using ITS and LSU sequences were performed against NCBIs GenBank nucleotide sequence database to identify the closest matching sequences, which were added to the sequences alignment. Sequences were aligned with MAFFT v. 7 (Katoh & Standley 2013) using the defaults settings and adjusted by hand in MEGA v. 6.06 (Tamura et al. 2013). To address the phylogenetic relationships among taxa, Bayesian inference (BI) using MrBayes v. 3.2.1 (Ronquist et al. 2012), and for maximum parsimony (MP) and neighbour-joining analysis with the Kimura 2-parameter and the HKY85 substitution model using PAUP v. 4.0b10 (Swofford 2003) were used as described by Crous et al. (2006). For parsimony analysis, alignment gaps were treated as a fifth character state with all characters unordered and of equal weight. The maximum parsimony analysis was performed with the heuristic search option with 100 random taxa additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. Other measures calculated included tree length, consistency index, retention index and rescaled consistency index (TL, CI, RI and RC, respectively). MrModelTest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model settings prior to the Bayesian analysis in MrBayes v. 3.2.1 (Ronquist et al. 2012). Nodal support was assessed by bootstrap analysis from 1 000 replicates. Bootstrap values (BS) equal or higher than 70 % were considered significant. Posterior probabilities for the Bayesian analysis (PP) were determined by calculating 50 % majority rule consensus tree.
Sequences derived in this study were deposited at GenBank, the alignments and trees in TreeBASE (http://treebase.org/treebase-web/home.html). The phylogenetic trees were edited using FigTree v. 1.4.0 and Adobe Illustrator CS5.1.
Morphology
Slide preparations were mounted in clear lactic acid from colonies sporulating on OA. Observations and photomicrographs were made with a Nikon SMZ1500 stereo-microscope, and with a Nikon eclipse Ni microscope, using a Nikon DS-U3 digital camera (Nikon, Tokyo, Japan) and NIS-Elements imaging software v. 4.20. Colony characters and pigment production were noted after 1 and 3 wk of growth on OA incubated at 25 °C. Colony colours (surface and reverse) were treated according to the colour chart of Rayner (1970).
RESULTS
Phylogeny
The LSU alignment was used to determine the generic relationships among Microdochium, Monographella and Idriella (Fig. 1), and the combined ITS, LSU, BTUB and RPB2 alignment (Fig. 2) to confirm species resolution in Microdochium.
Fig. 1.
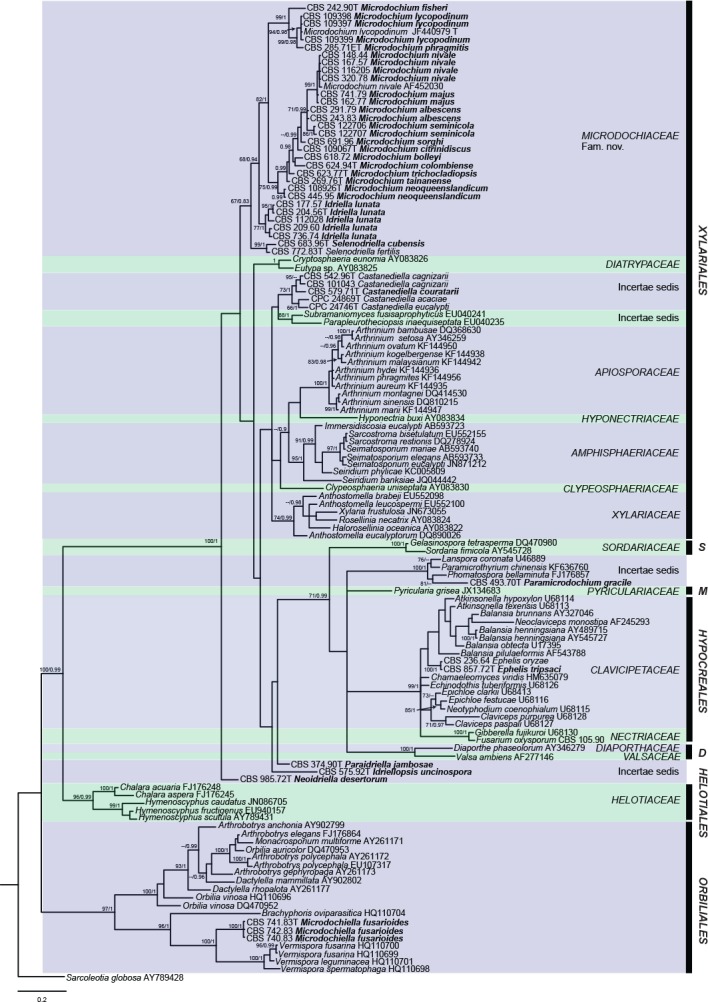
Maximum parsimony tree based on LSU data. Maximum parsimony bootstrap support values followed by Bayesian Posterior Probabilities are shown at the nodes. Orders and families are shown to the right of the tree and the scale bar indicates the number of changes. The tree was rooted with Sarcoleotia globosa. T = ex-type strain, ET = ex-epitype strain. D = Diaporthales, M = Magnaporthales, S = Sordariales.
Fig. 2.
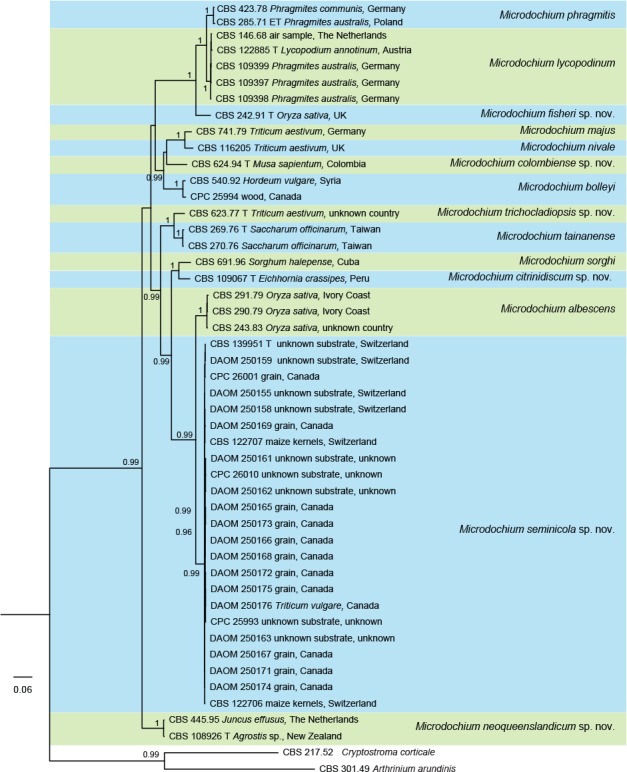
Bayesian phylogenetic tree inferred from the DNA sequence data from four loci (ITS, LSU, BTUB and RPB2) of Microdochium species. Bayesian posterior probabilities above 0.95 are indicated at the nodes and the scale bar indicates the number of expected mutations per site. Species names are shown to the right of the tree. The tree was rooted to Cryptostroma corticale (CBS 217.52) and Arthrinium arundinis (CBS 301.49). T = ex-type strain; ET = ex-epitype strain.
The LSU dataset consists of 124 aligned sequences, including the outgroup Sarcoleotia globosa and 898 characters, of which 467 constitute unique site patterns. Based on the results of MrModeltest, the GTR+I+G model with inverse gamma-distributed was selected as best fit model for Bayesian analyses. In the MP analyses 455 characters were constant, 111 were variable and parsimony uninformative while 332 were parsimony informative. A maximum of 1 000 equally most parsimonious trees were retained from this analysis (Tree length = 2 067, CI = 0.368, RI = 0.831 and RC = 0.305). The resulting MP tree is presented in Fig. 1 together with PP and BS values. The majority of the strains clustered in the Xylariales. However, Microdochium gracile CBS 493.70, is placed incertae sedis in the Sordariomycetes together with Lanspora coronata, Paramicrothyrium chinensis and Phomatospora bellaminuta. Microdochium tripsaci CBS 857.72 clusters in Clavicipitaceae (Hypocreales), and Microdochium fusarioides CBS 740.83, CBS 741.83 and CBS 742.83, clusters in Orbiliales.
The phylogenetic tree delimited seven families in Xylariales, one of which is described here as new (Microdochiaceae including Microdochium s.str., Idriella s.str. and Selenodriella), and six previously included families namely Apiosporaceae, Amphisphaeriaceae, Clypeosphaeriaceae, Diatrypaceae, Hyponectriaceae and Xylariaceae. Some species previously included in Idriella, viz. Idriella desertorum CBS 985.72, Idriella jambosae CBS 374.90 and Idriella uncinospora CBS 575.92, were placed incertae sedis in the Sordariomycetes phylogenetically distant from the type species of Idriella, I. lunata, and represent novel genera described in the taxonomy section. Idriella couratarii CBS 579.71 groups in a subclade in Xylariales with the recently described genus Castanediella (Crous et al. 2015), and is proposed here as a new combination.
Microdochium s.str. was analysed in more detail using multilocus data composed of 48 isolates including Arthrinium arundinis and Cryptostroma corticale as outgroups, and their aligned sequences of four genes, ITS, LSU, BTUB and RPB2. This dataset consisted in total of 2 955 characters (526 bp from the ITS, 831 bp from LSU, 772 bp from BTUB and 826 bp from RPB2) of which 871 constitutes unique site patterns. This phylogenetic tree (Fig. 2) delimited 14 species clades, seven of which represent novel species, described in the Taxonomy section below.
TAXONOMY
Orbiliales, incertae sedis
Microdochiella Hern.-Restr. & Crous, gen. nov. — MycoBank MB811866
Etymology. In reference to its morphological similarity with the genus Microdochium.
Type species. Microdochiella fusarioides (D.C. Harris) Hern.-Restr. & Crous.
Mycelium immersed and superficial, hyphae hyaline, septate. Conidiophores erect, hyaline, loosely branched. Conidiogenous cells polyblastic, terminal and intercalary, sympodial, denticulate, hyaline. Conidia solitary, dry but with a droplet of moisture at the mid-point of each conidium, hyaline, narrow-falcate, septate, truncate base and narrowly rounded at the apex. Chlamydospores subglobose to ellipsoidal, forming intercalary chains. Sexual morph unknown.
Microdochiella fusarioides (D.C. Harris) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811867
Basionym. Microdochium fusarioides D.C. Harris, Trans. Brit. Mycol. Soc. 84: 358. 1985.
Type details. UK, East Malling research station, on oospores of Phytophthora syringae, Oct. 1980, D.C. Harris (holotype IMI 281715).
Description & illustration — See Harris (1985).
Specimens examined. UK, East Malling research station, on oospores of Phytophthora syringae, Oct. 1980, D.C. Harris, living ex-type culture CBS 741.83; other living cultures CBS 740.83 and CBS 742.83.
Notes — In the phylogenetic tree three strains of M. fusarioides formed a well-supported clade related but clearly separated from Vermispora in Orbiliales. Asexual morphs in this fungal order are characterised by holoblastic conidiogenesis, absence of yeast-like budding, and some of them can produce trapping organs, although non-predacious and freshwater fungi are also frequently found in Orbiliales (Li et al. 2005, Chen et al. 2007, Yu et al. 2011). Vermispora and Microdochiella are similar morphologically, but they have different ecological preferences. The genus Vermispora includes five species isolated from soil, dead leaves and eggs of nematodes (Chen et al. 2007). Although the genus is apparently monophyletic, no live material of the type species, V. grandispora, exists. Here we introduce Microdochiella to include one atypical microdochium-like species growing on oospores of Phytophthora syringae (Harris 1985). Phylogenetically Microdochiella is clearly distinct from Vermispora. The three strains of M. fusarioides remained sterile in culture.
Sordariomycetes, Hypocreales, Clavicipitaceae
Ephelis tripsaci (D. Mulder & Arx) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811868; Fig. 3
Fig. 3.
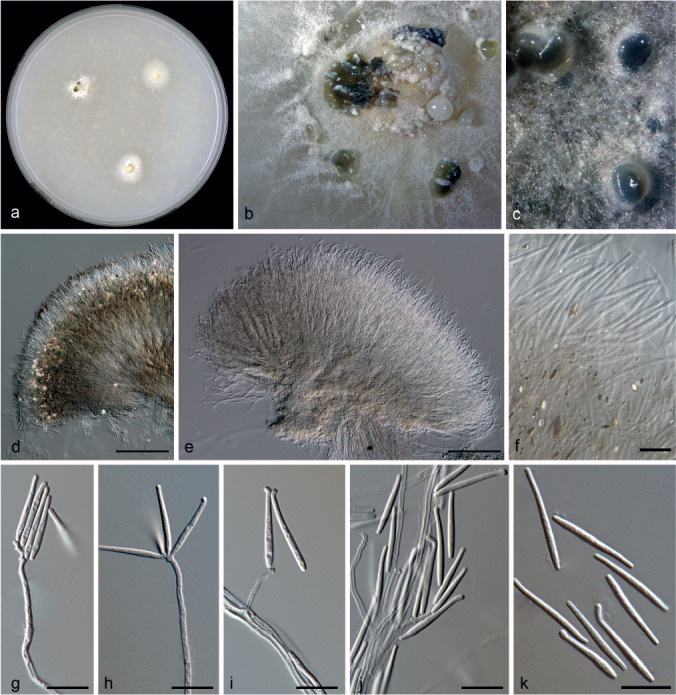
Ephelis tripsaci (from ex-type, CBS 857.72). a. Colony on OA at 25 °C in 3 wk; b, c. colony overview; d, e. sporodochia; f. extracellular pigments and crystals; g–j. conidiogenous cells; k. conidia. — Scale bars: d, e = 100 μm; f–k = 10 μm.
Basionym. Microdochium tripsaci D. Mulder & Arx, Sydowia 34: 32. 1981.
Mycelium immersed or superficial, hyphae branched, septate, hyaline. Sporodochia white buff to olivaceous black, 276–510 μm diam, solitary to aggregated, often confluent at base, textura intricata-epidermoidea, 1.5–4 μm diam; hymenium with hyaline to pale brown cells, dark sporodochia with crystals (white to yellow) and extracellular brown pigments (umber to chestnut). Conidiophores branched, hyaline, smooth. Conidiogenous cells holoblastic, sympodial, terminal or lateral on aerial mycelium, straight or flexuous, cylindrical, 13–86 × 1–2 μm, hyaline. Conidia in whorls at the tips of conidiogenous cells, acicular, vermiform-subulate or obclavate, 12–22.5 × 1.5–3 μm, unicellular, hyaline, smooth-walled, apically rostrate and curved, base truncate, 1 μm diam.
Culture characteristics — Colonies on OA reaching 12–14 mm diam in 3 wk, velvety to powdery, white, margin with rhizoids. Sporodochia formed after 2 wk near the inoculum, vinaceous buff to grey olivaceous.
Specimen examined. SRI LANKA, on leaf sheath in Tripsacum laxum, Oct. 1972, D. Mulder (holotype CBS H-22144; living culture ex-type CBS 857.72).
Notes — The isolate CBS 857.72 groups in a clade with Ephelis oryzae CBS 236.64 and other members of Clavicipitaceae (Fig. 1). Ephelis tripsaci was initially included in Microdochium. Nevertheless, the isolate CBS 857.72 fits with the Ephelis concept, based on molecular and morphological data. Blast search of ITS resulted in a 99 % of similarity with AB038564 of Ephelis japonica, CBS 236.64 of Ephelis oryzae and several other unidentified Ephelis spp. Conidial morphology in E. tripsaci is slightly different from E. japonica and E. oryzae, having shorter and wider conidia (in E. japonica they are 20–30 × 0.7–1 μm, and in E. oryzae 20–35 × 1 μm, in E. tripsaci 12–22.5 × 1.5–3 μm). Ephelis has been reported as asexual morph occurring in different genera in Clavicipitaceae (Hypocreales) mainly in Atkinsonella, Balansia, Myriogenispora and Nigrocornus (Kuldau et al. 1997, White et al. 2003, Seifert et al. 2011). Phylogenetic studies demonstrated that species of Atkinsonella, Balansia and Myriogenispora with Ephelis asexual states form a monophyletic clade (Kuldau et al. 1997). Further taxonomic studies are clearly needed on these genera.
Sordariomycetes, incertae sedis
Paramicrodochium Hern.-Restr. & Crous, gen. nov. — MycoBank MB811869
Etymology. Named after its morphological similarity to, but being distinct from, Microdochium.
Type species. Paramicrodochium gracile (Mouch. & Samson) Hern.-Restr. & Crous.
Mycelium immersed and superficial, hyphae hyaline, septate, smooth. Conidiophores slightly differentiated, branched, hyaline. Conidiogenous cells polyblastic, occasionally monoblastic, terminal and intercalary, sympodial, denticulate, cylindrical, lageniform, straight or curved. Conidia solitary, dry, hyaline, unicellular, smooth, filiform to falcate, straight or curved, truncate at base, tapering towards the apex. Sexual morph unknown.
Paramicrodochium gracile (Mouch. & Samson) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811870; Fig. 4
Fig. 4.
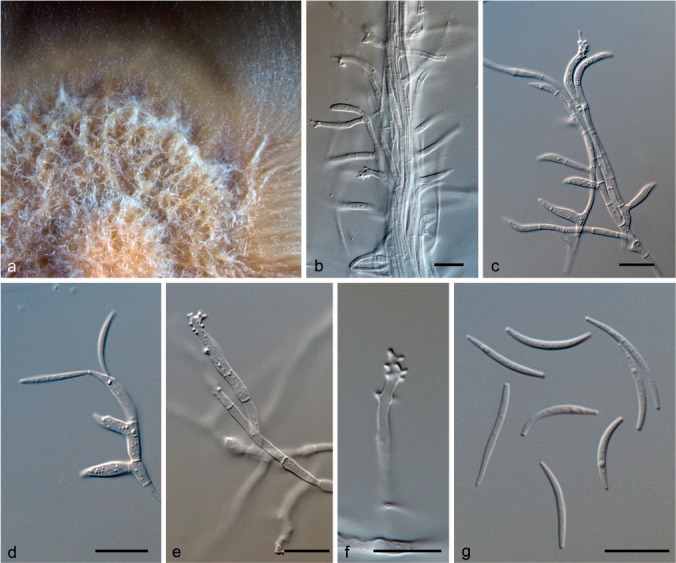
Paramicrodochium gracile (from ex-type, CBS 493.70). a. Colony on OA at 25 °C in 4 wk; b–f. conidiogenous cells; g. conidia. — Scale bars: b–g = 10 μm.
Basionym. Microdochium gracile Mouch. & Samson, Rev. Mycol. (Paris) 37: 270. 1973.
Mycelium immersed and superficial, hyphae septate, hyaline. Conidiogenous cells mainly terminal, mono- and polyblastic, denticulate, straight or curved, cylindrical to slightly inflated in the median region, 7–31.5 × 1.5–3 μm, hyaline, smooth. Conidia acicular, falcate, 11–18 × 0.8–1.5 μm, unicellular, hyaline, apex pointed, base truncate.
Culture characteristics — Colonies on OA 20 mm diam in 4 wk. Flat, aerial mycelium absent, with concentric rings, flesh to white at the periphery, margin entire; reverse saffron. On MEA, 25–30 mm diam after 4 wk. Convex, funiculose, peach, margin fimbriate; reverse scarlet.
Specimen examined. THE NETHERLANDS, Baarn, Groeneveld, isolated from rabbit dung, 1970, G.S. de Hoog, (CBS H-22138, living culture ex-type CBS 493.70 (as Microdochium gracile).
Notes — The strain CBS 493.70 grouped incertae sedis (Sordariomycetes) in a clade distant from Xylariales. Paramicrodochium is introduced here to accommodate a microdochium-like taxon isolated from a rabbit dung sample collected in The Netherlands. According to the phylogenetic analysis this taxon does not cluster with Microdochium s.str. Paramicrodochium gracile was placed as the sister clade of Lanspora coronata, Paramicrothyrium chinensis and Phomatospora bellaminuta. These obscure fungi are sexual morphs without any known asexual morph. Paramicrothyrium chinenses is a fungus that grows on dead leaves found in China and produces thyrothecial ascomata (Wu et al. 2011). Lanspora coronata is a marine species that grows on driftwood collected in Seychelles (Hyde & Jones 1986). Phomatospora bellaminuta was isolated from senescent culms of Juncus roemerianus in North Carolina (USA) and also considered a marine fungus (Kohlmeyer et al. 1995). Additional samples are needed in order to assess the higher taxonomical rank and to understand the ecology and geographic distribution of species in this clade.
Sordariomycetes, Xylariales
Microdochiaceae Hern.-Restr., Crous & J.Z. Groenew., fam. nov. — MycoBank MB811871
Saprobic, endophytic or pathogenic; on leaves, seeds and soil. Sexual morph. Stroma present or absent. Ascomata perithecial. Asci cylindrical, oblong, clavate, with amyloid funnel-shaped apical ring and 8 biseriate or uniseriate ascospores. Ascospores ellipsoid or oblong, fusoid, hyaline to pale brown. Asexual morph. Conidiomata if present, sporodochial. Conidiophores solitary or aggregated, mono- or biverticillate. Conidiogenous cells solitary or in whorls, polyblastic, sympodial, denticulate, cylindrical often ampulliform, lageniform with elongated necks and minute annellides from percurrent proliferations, hyaline to pale brown. Conidia lunate, oblong, fusiform or cylindrical, straight or curved, hyaline, flattened at base. Chlamydospores if present, brown.
Type genus. Microdochium Syd.
Included genera — Idriella, Microdochium and Selenodriella.
Idriella P.E. Nelson & S. Wilh., Mycologia 48: 550. 1956
Mycelium immersed and superficial, hyphae hyaline to brown, septate, smooth. Conidiophores brown, non-septate. Conidiogenous cells polyblastic, terminal, denticulate, lageniform to cylindrical. Conidia dry, in heads, hyaline, unicellular, smooth, lunate, curved. Chlamydospores brown, uni- or pluricellular. Sexual morph unknown.
Type species. Idriella lunata P.E. Nelson & S. Wilh.
Idriella lunata P.E. Nelson & S. Wilh., Mycologia 48: 550. 1956 — Fig. 5
Fig. 5.
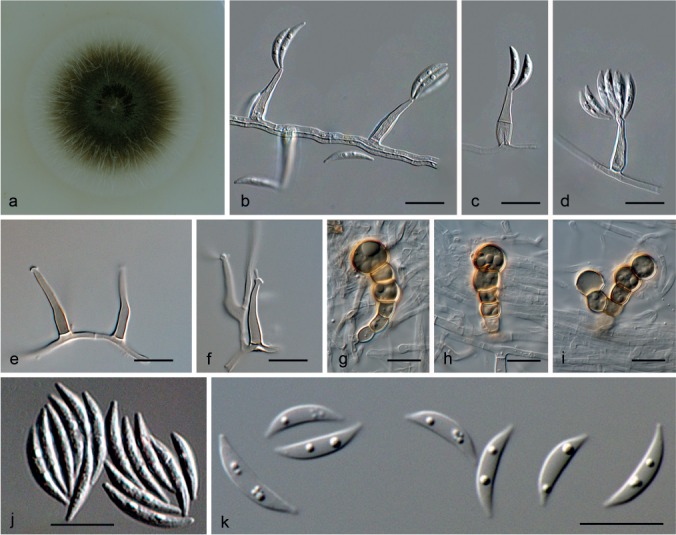
Idriella lunata (a–d, j from ex-type CBS 204.56; e from CBS 177.57; g–i, k from CBS 404.78). a. Colony overview on OA at 25 °C in 3 wk; b–f. conidiogenous cells; g–i. chlamydospores; j, k. conidia. — Scale bars: b–k = 10 μm.
Specimens examined. JAPAN, Kamakura, unknown substrate, Dec. 1974, K. Takano, living culture CBS 736.74. – THE NETHERLANDS, isolated from soil, Oct. 1960, J.C. Went, living culture CBS 209.60. – UNKNOWN, unknown substrate, 12 Jan. 1957, P.E. Nelson, living culture CBS 177.57. – USA, Santa Clara, California, on diseased roots of Fragaria chiloensis, Sept. 1950, P.E. Nelson, living culture ex-type CBS 204.56.
Notes — Idriella lunata was introduced for a fungus growing on infected roots on Fragaria chiloensis (Nelson & Wilhelm 1956) and the genus currently comprises 30 species (Matsushima 1971, Von Arx 1981, Castañeda-Ruiz & Kendrick 1991, Rodrigues & Samuels 1992). Nevertheless, our phylogenetic analyses suggest that Idriella is a monotypic genus, and species formerly described in this genus as I. desertorum, I. jambosae and I. uncinospora, depict new genera. Although the phylogenetic position of these new genera is still unclear, they do not belong to the Microdochiaceae, but appear as members of Sordariomycetes with uncertain position (Fig. 1).
Microdochium Syd., Ann. Mycol. 22: 267. 1924
=Monographella Petr., Ann. Mycol. 22: 144. 1924.
=Griphosphaerella Petr., Ann. Mycol. 25: 209. 1927.
=Gloeocercospora D.C. Bain & Edgerton, Trans. Brit. Mycol. Soc. 57: 358. 1971.
=Gerlachia W. Gams & E. Müll., Netherlands J. Agric. Sci. 86: 49. 1980.
Type species. Microdochium phragmitis Syd.
Mycelium immersed, branched, septate, hyphae hyaline to pale brown. Sporodochia, if present, epidermal, subepidermal, erumpent through stomata, through rupture of the outer epidermal wall and cuticle, or by specialized egression hyphae through the outer epidermal wall; hyaline, pseudoparenchymatic, spreading after egress. Conidiophores more or less verticillate, often slightly differentiated, reduced to conidiogenous cells, hyaline, smooth. Conidiogenous cells holoblastic, discrete, hyaline, smooth, solitary or aggregated in small sporodochia. Two kinds: with sympodial proliferation, cylindrical or slightly tapering, or clavate, denticulate with one or more apical denticles. Or with percurrent proliferation (annellidic), subcylindrical, obpyriform, ampulliform to lageniform. Conidia dry or in slimy mass, unicellular or multiseptate, hyaline, smooth, lunate, falcate, fusiform, filiform, obovoid or subpyriform, straight or curved, apex rounded, base flattened. Sometimes the conidia originate directly from hyphae. Chlamydospores terminal or intercalary, solitary, in chains or grouped in clusters, brown. Sexual morph monographella-like, on natural substrate. Ascomata perithecial, immersed, subepidermal, solitary or in groups, pale brown to black, globose, subglobose to oval with central, papillate and often acute ostiole, ostioles usually more distinctly pigmented than the perithecial body, filled with slightly clavate periphyses. Peridium brown, thin-walled, thickened and darker around the ostiole, in view face textura angularis-epidermoidea. Paraphyses filamentous, apically free, thin-walled. Asci unitunicate, oblong to clavate with 8 bi- to multiseriate ascospores, apex with an amyloid, refractive, flat, funnel-shaped ring. Ascospores clavate, fusoid or oblong, hyaline to brownish, straight or curved, smooth and septate.
Microdochium albescens (Thüm.) Hern.-Restr. & Crous, comb. nov. — MycoBank MB812167
Basionym. Metasphaeria albescens Thüm., Die Pilze der Reispflanze 12: 5. 1889.
≡ Griphosphaerella albescens (Thüm.) Arx, Gen. Fungi Sporul. Cult., Edn 3 (Vaduz): 174. 1981.
≡ Monographella albescens (Thüm.) V.O. Parkinson, Sivan. & C. Booth, Trans. Brit. Mycol. Soc. 76: 64. 1981.
=Metasphaeria oryzae-sativae Hara, Diseases of the Rice Plant (Japan): 151. 1918.
=Rhynchosporium oryzae Hashioka & Yokogi, Contrib. Lab. Plant Disease Sci. Fac. Agric. Gifu Univ. 6: 51. 1955.
≡ Gerlachia oryzae (Hashioka & Yokogi) W. Gams, in Gams & Müller, Neth. J. Pl. Path. 86: 50. 1980.
≡ Microdochium oryzae (Hashioka & Yokogi) Samuels & I.C. Hallett, Trans. Brit. Mycol. Soc. 81: 481. 1983.
=Micronectriella pavgii R.A. Singh, Friesia 11: 238. 1978.
Microdochium citrinidiscum Hern.-Restr. & Crous, sp. nov. — MycoBank MB811872; Fig. 6
Fig. 6.
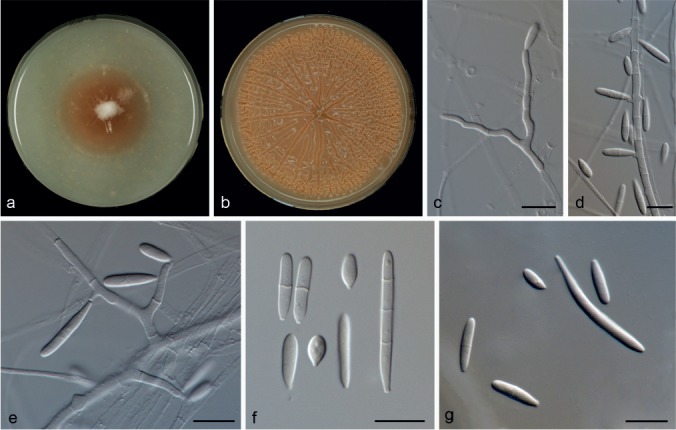
Microdochium citrinidiscum (from ex-type, CBS 109067). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c–e. conidiogenous cells; f, g. conidia. — Scale bars: c–g = 10 μm.
Etymology. Latin Citrinus- meaning orange and discus- disk; in reference to their orange slice colony appearance.
Mycelium mostly immersed, hyphae hyaline, septate, smooth, 1.5–4 μm wide. Conidiophores undifferentiated. Conidiogenous cells terminal or intercalary, mono- or polyblastic, denticulate, cylindrical, 11–29 × 1.5–2 μm. Conidia cylindrical, clavate, obovoid, 0–3-septate, 7–31 × 2–3 μm, base usually flattened 0.5–1 μm. Sometimes born directly from the mycelial hyphae. Chlamydospores not observed.
Culture characteristics — Colonies on OA 40 mm diam after 1 wk, centre aerial mycelium cottony, white, periphery scarce aerial mycelium, saffron, margin diffuse, reverse saffron, no exudate or soluble pigment produced. After 3 wk radially folded to rugose, shiny, dark saffron, margin diffuse, reverse cinnamon.
Specimen examined. PERU, Ucayali, Yarinacocha, Isla de Amor, on leaf of Eichhornia crassipes, 21 Oct. 1998, H.C. Evans, isolated by D.H. Djeddour (No. W1916f) (holotype CBS H-22132; living culture ex-type CBS 109067).
Notes — This species forms a clade with CBS 691.96 (listed in the CBS database as M. sorghi, not an ex-type culture). Unfortunately, the latter isolate remains sterile and only produces black sclerotia in culture. Microdochium sorghi is a widespread fungus that causes zonate leaf spots on Sorghum and other species of Poaceae. Microdochium sorghi is different from M. citrinidiscum in having larger conidia (50–125 × 1–3 μm, in culture) with up to 10 septa. Furthermore, it is characterized by producing sclerotial bodies in both natural substratum and in culture (Von Arx 1987, Braun 1995). In contrast, M. citrinidiscum is only known from Peru growing on leaves of Eichhornia crassipes, has smaller conidia (7–31 × 2–3 μm) with up to 3 septae, and lacks sclerotia in culture.
Microdochium colombiense Hern.-Restr. & Crous, sp. nov. — MycoBank MB811873; Fig. 7
Fig. 7.
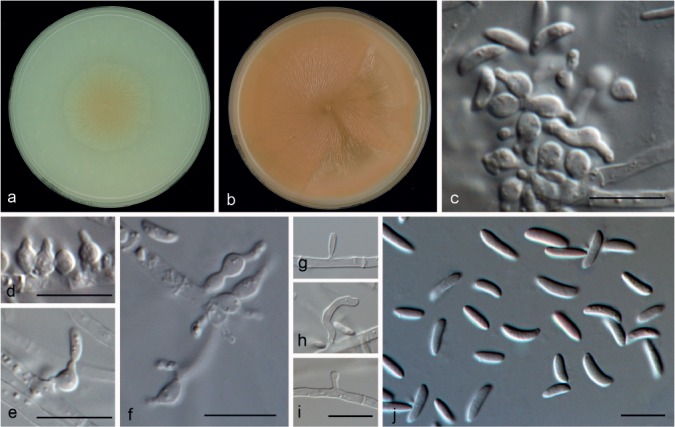
Microdochium colombiense (from ex-type, CBS 624.94). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c–f. conidiogenous cells ampulliform with percurrent proliferations; g–i. conidiogenous cells cylindrical to clavate; j. conidia. — Scale bars: c–j = 10 μm.
Etymology. Named after the country where this fungus was collected, Colombia.
Mycelium mostly immersed; hyphae hyaline, septate, 1.5–2.5 μm wide. Conidiogenous cells of two types: some polyblastic, ampulliform, with percurrent proliferations, 5–11.5 × 2.5–3.5 μm, neck up to 4.5 μm long, 1–1.5 μm wide, others cylindrical up to 13 μm long, 1–2 μm wide. Conidia lunate, fusiform, allantoid or reniform, straight or curved, 5–8 × 1.5–2.5 μm, 0(–1)-septate, base truncate. Sometimes produced directly on mycelial hyphae. Chlamydospores not observed.
Culture characteristics — Colonies on OA 40 mm diam after 1 wk, flat, salmon, no exudate or soluble pigment produced, margin diffuse or entire; reverse saffron. After 3 wk 90 mm diam, flat, orange peach, radially striate.
Specimen examined. COLOMBIA, on Musa sapientum, Jan. 1995, L. Verbruggen (holotype CBS H-22133; living culture ex-type CBS 624.94).
Notes — Microdochium colombiense forms a sister clade to M. nivale and M. majus, and is morphologically distinguished from those species by their conidial morphology. Microdochium colombiense has smaller conidia (5–8 × 1.5–2.6 μm) than those of M. majus (6–15 × 2–4 μm) and M. nivale (5–36 × 2–4.5 μm). Furthermore, conidia in M. colombiense are mostly aseptate (rarely 1-septate), while in M. majus and M. nivale conidia are mostly 3-septate (up to 10- or 7-septate, respectively). Although M. colombiense resembles M. neoqueenslandicum, they are phylogenetically distinct (Fig. 2). Additionally, the colony growth rate at 30 °C after 1 wk was about 10 mm in M. colombiense (CBS 624.94) and 35–37 mm in M. neoqueenslandicum (CBS 445.95 and CBS 108926) under the same conditions. At lower temperatures (12, 18 and 24 °C) the grow rate was similar for both species.
Microdochium consociatum (Rehm) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811967
Basionym. Leptosphaeria consociata Rehm, Hedwigia Beibl.: 149. 1896.
= Monographella consociata (Rehm) O.E. Erikss. & J.Z. Yue, Mycotaxon 38: 205. 1990.
Microdochium fisheri Hern.-Restr. & Crous, sp. nov. — MycoBank MB811874; Fig. 8
Fig. 8.
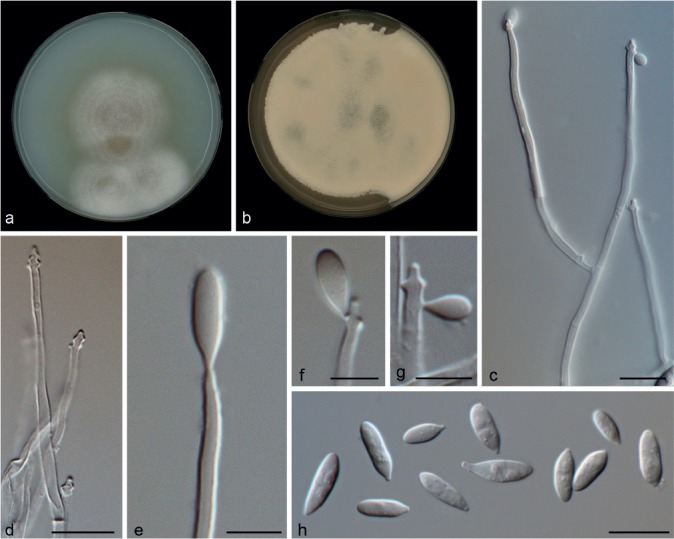
Microdochium fisheri (from ex-type, CBS 242.91). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c, d. conidiophores; e–g. conidiogenous cells; h. conidia. — Scale bars: c–h = 10 μm.
Etymology. Named in honour of P.J. Fisher, who collected this fungus in the UK.
Mycelium superficial and immersed; hyphae hyaline, branched, septate. Conidiophores slightly differentiated, bifurcate, hyaline, smooth. Conidiogenous cells terminal, sympodial, denticulate, cylindrical, 19–60 × 1.5–2 μm, hyaline, smooth. Conidia solitary, dry, fusiform, obovoid, subpyriform, to clavate, 7–12 × 3–4 μm, 0–1-septate, hyaline, tapering to a subtruncate hilum; hilum unpigmented. Chlamydospores not observed.
Culture characteristics — Colonies on OA 45–50 mm diam after 1 wk, powdery to velvety, aerial sporulation, centre salmon, periphery peach, margin entire; reverse salmon.
Specimen examined. UK, on stem of Oryza sativa (greenhouse-grown plant, endophytic), June 1990, P.J. Fisher (holotype CBS H-22142; living culture ex-type CBS 242.90).
Notes — Isolate CBS 242.90 forms a separated branch as the sister clade of M. phragmites and M. lycopodinum. Originally, the isolate CBS 242.90 was identified as Arthrobotrys foliicola (no ex-type isolate available) which is morphologically similar, but A. foliicola was originally described with pale brown, sympodial, and nodose proliferations in the conidiophores, terminal and intercalary, swollen conidiogenous cells, hyaline conidia with brown septa, appearing pale brown in mass (Matsushima 1975). Microdochium fisheri is different since it has hyaline, tapering conidiophores, with denticulate conidiogenous cells and hyaline conidia, without pigment at the septa, appearing salmon in mass.
Microdochium fusariisporum (Ellis & Everh.) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811968
Basionym. Rhopographus fusariisporus Ellis & Everh., Erythea 2: 23. 1894.
≡ Exarmidium fusariisporum (Ellis & Everh.) Theiss. & Syd., Ann. Mycol. 13: 424. 1915.
≡ Monographella fusariispora (Ellis & Everh.) M.E. Barr, Mycotaxon 46: 63. 1993.
Microdochium lycopodinum (Jaklitsch, Siepe & Voglmayr) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811969; Fig. 9, 10
Fig. 9.
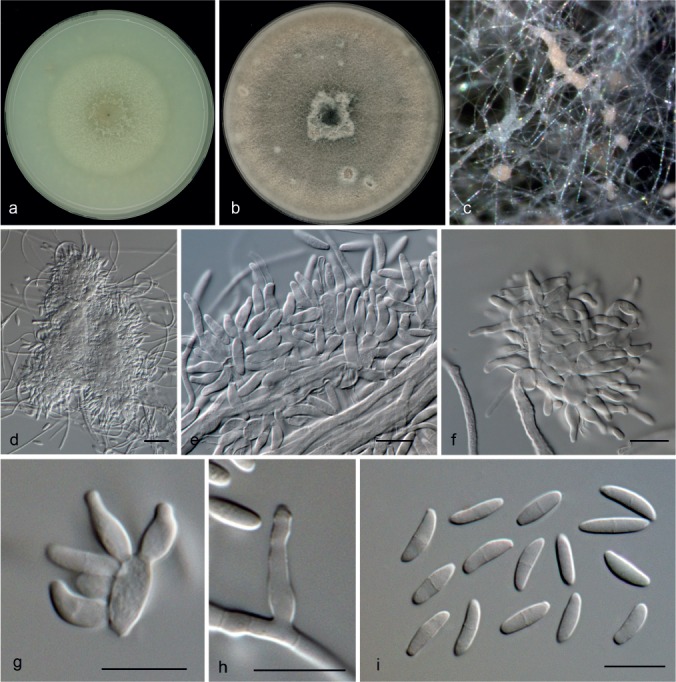
Microdochium lycopodinum (from CBS 109398). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c. colony overview; d–f. aggregated conidiophores; g, h. conidiogenous cells; i. conidia. — Scale bars: d = 25 μm; e–i = 10 μm.
Fig. 10.
Microdochium lycopodinum (from CBS 109399). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c. colony overview; d. sporodochia with brown mycelium; e, f. aggregated conidiophores; g, h. conidiogenous cells; i. conidia. — Scale bars: d = 100 μm; e–i = 10 μm.
Basionym. Monographella lycopodina Jaklitsch, Siepe & Voglmayr, Fung. Diversity 52: 86. 2012.
Description of sexual morph — Jaklitsch & Volgmayr (2012).
Mycelium immersed and superficial, hyphae hyaline to pale brown, septate, smooth or verruculose, 1.5–6 μm diam. Conidiophores more or less mono- to biverticillate, metulae doliiform to clavate, aggregated in slimy masses in the aerial mycelium often reduced to conidiogenous cells born directly from the hyphae. Conidiogenous cells holoblastic, with percurrent proliferations, ampulliform to lageniform, subcylindrical, 4–12 × 2.5–3.5 μm. Conidia hyaline, fusiform or with one side straighter than the other, lunate, 8–15.5 × 2.5–4 μm, 0–1-septate, truncate base, rounded apex. Some conidia are borne directly on the mycelial hyphae. Chlamydospores not observed. Sclerotia superficial on the agar, brown to dark brown, textura angularis.
Culture characteristics — Colonies on OA reaching 50–54 mm after 1 wk. White cottony, lanose to flocosse, buff to rosy buff, margin effuse. After 3 wk with aerial mycelium profuse, olivaceous grey with some white to saffron patches, aerial sporulation in aggregated slimy masses, rosy buff to umber; reverse greenish black with some white patches.
Specimens examined. AUSTRIA, Oberösterreich, St. Willibald, Salletwald, on leaves of Lycopodium annotinum, 19 July 2009, H. Voglmayr (living culture ex-type CBS 125585). – GERMANY, Konstanz, on Phragmites australis, May 1997, W. Leibinger, living cultures CBS 109397, CBS 109398, CBS 109399. – THE NETHERLANDS, Sellingen, isolated from an air sample, Dec. 1967, A. Kikstra (No. 1062), living culture CBS 146.68.
Notes — The clade M. lycopodinum is represented by five isolates with M. phragmitis as sister clade. The ex-type culture of M. lycopodinum, CBS 122885, and CBS 146.68 remained sterile. Isolate CBS 109397 was morphologically degenerated, as colonies lacked aerial mycelium and sporodochia, conidiogenous cells were scarce and small, and sclerotial bodies were present. The other two strains, CBS 109398 and CBS 109399 (Fig. 9, 10), showed colonies with abundant aerial mycelium, producing superficial sporodochial-like structures, with verticillate conidiophores and abundant conidiogenous cells and conidia. Microdochium lycopodinum was originally described as sexual morph growing in leaves of Lycopodium annotium in Austria and Germany (Jaklitsch & Volgmayr 2012). Nevertheless, original cultures were sterile and no asexual morph has been reported. According to our phylogenetic analysis based in four genes (Fig. 2), isolates CBS 146.68, CBS 109397, CBS 109398 and CBS 109399 represent the same phylogenetic species as M. lycopodinum. Here we newly describe the asexual morph of M. lycopodinum.
Microdochium maydis (E. Müll. & Samuels) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811970
Basionym. Monographella maydis E. Müll. & Samuels, Nova Hedwigia 40: 114. 1984.
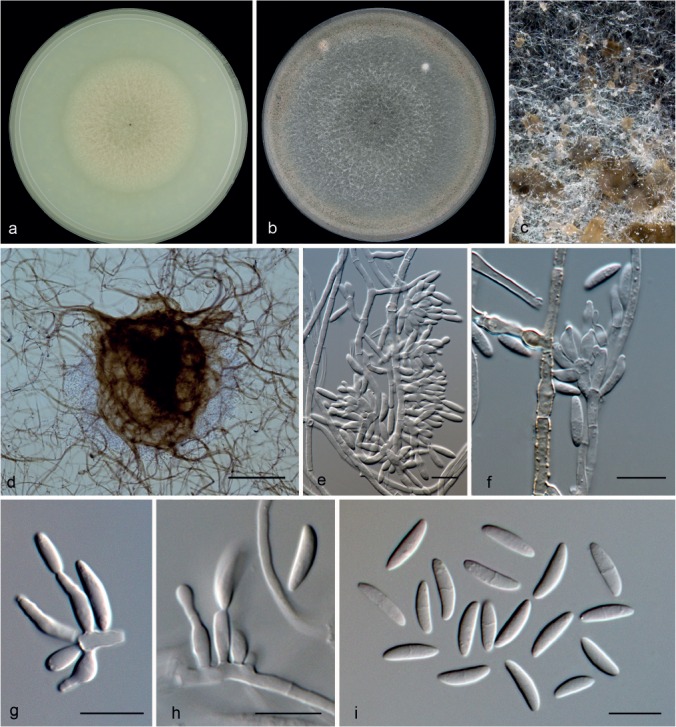
Microdochium neoqueenslandicum Hern.-Restr. & Crous, sp. nov. — MycoBank MB811875; Fig. 11
Fig. 11.
Microdochium neoqueenslandicum (from ex-type, CBS 108926). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c. colony overview; d. sporodochia; e–h. conidiophores with conidiogenous cells; i. conidia. — Scale bars: d = 25 μm; e–i = 10 μm.
Etymology. Named after its resemblance to Microdochium queenslandicum.
Mycelium immersed or superficial, hyphae hyaline, septate, smooth. Sporodochia slimy, orange. Conidiophores more or less mono- or biverticillate, metulae clavate to cylindrical. Conidiogenous cells polyblastic, with percurrent proliferations, ampulliform, lageniform to subcylindrical, 4.5–10 × 2–3.5 μm, neck up to 4 μm long, 1–1.5 μm diam, solitary or in whorls. Conidia lunate, allantoid, curved, with one side straighter than the other, 0(–1)-septate, 4–9 × 1.5–3 μm, base flattened. Sometimes produced directly on the mycelial hyphae. Chlamydospores not observed.
Culture characteristics — Colonies on OA 40–47 mm diam after 1 wk, centre flat, creamy, with concentric rings, peach to salmon, periphery with cottony aerial mycelium, white, margin diffuse, entire. After 3 wk with concentric rings scarlet of sporodochia, alternate with a dense zone of white, cottony aerial mycelium, exudate hyaline. No aerial mycelium nor sporodochia were observed in degenerated cultures.
Specimens examined. NEW ZEALAND, Waihi, Waihi Golf Club, on Agrostis sp., 24 Jan. 2000, A. Ellis (Holotype CBS H-22136; living culture ex-type CBS 108926). – THE NETHERLANDS, Brecklenkamp, Twente, on Juncus effusus, 6 Apr. 1995, E. Brouwer, living culture CBS 445.95.
Notes — Microdochium neoqueenslandicum is represented by two isolates, CBS 108926 and CBS 445.95, and clustered basal to other Microdochium species (Fig. 2). Microdochium neoqueenslandicum is distinct from M. queenslandicum by having shorter and wider conidia (7.5–11 × 1.8–2.2 μm in M. queenslandicum). Microdochium queenslandicum is only known from a forest soil sample collected in Australia. Unfortunately, no living material of M. queenslandicum was available for study. In our phylogenetic tree M. neoqueenslandicum is represented by two strains isolated from grasses, Agrostis sp. and Juncus effuses, the former from New Zealand and the latter from the Netherlands, suggesting that this species has a wide distribution. The isolate CBS 445.95 was macro- and micromorphologically degenerated, since colonies lacked aerial mycelium and sporodochia, and conidiogenous cells were scarce and smaller in size.
Microdochium opuntiae (Ellis & Everh.) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811971
Basionym. Sphaerella opuntiae Ellis & Everh., J. Mycol. 4: 97. 1888.
≡ Mycosphaerella opuntiae (Ellis & Everh.) Dearn., Bull. New York State Mus. Nat. Hist. 205: 55. 1919.
≡ Monographella opuntiae (Ellis & Everh.) Arx, Trans. Brit. Mycol. Soc. 83: 374. 1984.
= Gloeosporium lunatum Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 43: 82. 1891.
≡ Fusarium lunatum (Ellis & Everh.) Arx, Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., sect. 2. 51: 101. 1957.
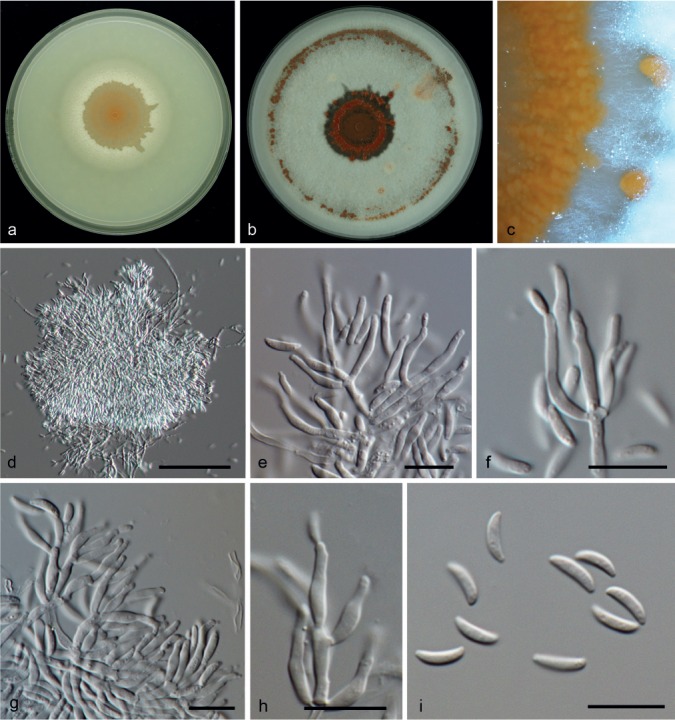
Microdochium phragmitis Syd., Ann. Mycol. 22: 267. 1924 — Fig. 12, 13
Fig. 12.
Microdochium phragmitis (from ex-epitype, CBS 285.71). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c. colony overview; d–h. conidiogenous cells; i. conidia. — Scale bars: d–i = 10 μm.
Fig. 13.
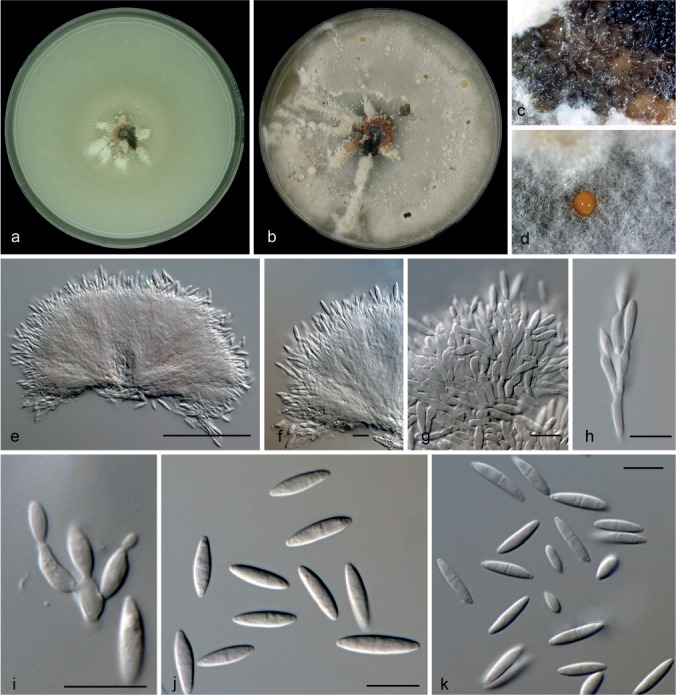
Microdochium phragmitis (from CBS 423.78). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c, d. colony overview with orange, slimy sporodochia; e–g. sporodochia; h, i. conidiophores with conidiogenous cells; j, k. conidia. — Scale bars: e = 50 μm; f–k = 10 μm.
Description based on CBS 285.71 after 1 wk on OA at 24 °C. Mycelium superficial and immersed, hyphae hyaline, septate, 1–2 μm wide. Conidiogenous cells terminal, sympodial, denticulate, hyaline, smooth, cylindrical to clavate, sometimes navicular, 6–24 × 1.5–3 μm. Conidia dry, solitary, fusiform, navicular or clavate, 0–1-septate, 10–14.5 × 2–3 μm, guttulate. Chlamydospores not observed.
Culture characteristics — Colonies on OA 37 mm diam after 1 wk. Floccose, white in the centre, sparse aerial mycelium, buff to the periphery, margin effuse; reverse buff. After 3 wk velvety.
Specimens examined. GERMANY, Berlin, Brandenburg, on leaves of Phragmites communis, Nov. 1919, H. Sydow (holotype K-IMI 193888; Sydow, Mycotheca germanica 2250). – POLAND, Bialowiesza National Park, on Phragmites australis, Sept. 1966, W. Gams (epitype designated here CBS H-22135, MBT200934, living culture ex-epitype CBS 285.71). – THE NETHERLANDS, Nijkerk, on a leaf of Phragmites australis, unknown date, P. Reinecke, CBS H-22134, CBS 423.78 living culture.
Notes — The ex-epitype strain CBS 285.71 and CBS 423.78 clustered together (1 PP) in the combined tree (Fig. 2). Nevertheless, the strain CBS 423.78 (Fig. 13) differs from the ex-epitype strain CBS 285.71 (Fig. 12) in its colony appearance and micro-morphological characters. CBS 423.78 produces pionnotal sporodochia, conidiogenous cells lageniform with annellidic percurrent proliferations, conidia produced in slimy mass, fusiform, 12.5–16 × 3–3.5 μm and obovoid, unicellular, 4.5–8.5 × 2–4 μm. Molecularly those strains were very similar, their ITS sequences were identical and the other genes only differ in 5, 3 and 1 bp in their BTUB, RPB2 and LSU sequences, respectively, which would indicate they are the same phylogenetic species. Considering the polymorphism in Microdochium and the difficulties to delimit species in this group we refer to CBS 423.78 as M. phragmites.
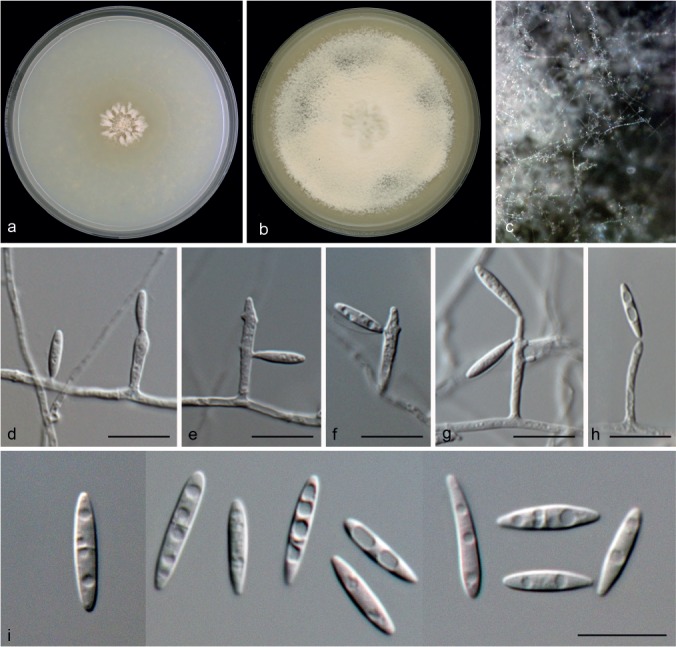
Microdochium seminicola Hern.-Restr., Seifert, Clear & B. Dorn, sp. nov. — MycoBank MB812168; Fig. 14
Fig. 14.

Microdochium seminicola (from ex-type, CBS 139951). a. Colony on OA at 25 °C in 3 wk; b. colony overview of the sporodochia; c. colony overview of the ascomata; d. sporodochia; e–g. conidiophores with conidiogenous cells and conidia attached; h. conidia; i. ascomata; j–l. asci (k and m in Melzer’ reagent); m. ascus ring in Melzer’s reagent; n. ascospores — Scale bars: d–h; j–n = 10 μm; i = 50 μm.
Etymology. Latin seminicola- meaning growing on seeds.
Mycelium immersed and superficial, hyphae hyaline, septate, smooth. Sporodochia slimy, peach, minute to essentially pionnotal. Conidiophores more or less biverticillate, metulae doliiform. Conidiogenous cells solitary or in whorls, ampulliform to lageniform, with percurrent proliferations, 5–11 × 3–4 μm, the neck 1–1.5 μm wide. Conidia cylindrical to fusiform, straight or curved, 19–54 × 3–4.5 μm, (0–)3(–5)-septate, hyaline, tapering at the apex, occasionally curved at the tip, base usually flattened. Chlamydospores not observed. Sexual morph. Perithecia submerged or superficial on the agar, solitary or in groups, spherical to subspherical, 110–149 μm diam, pale brown to brown, textura angularis-epidermoidea. Paraphyses filiform, hyaline. Asci basal, 41–66.5 × 7.5–11 μm, oblong, narrowly clavate, fusiform, with 8 ascospores, short stipe and amyloid, funnel-shaped apical ring. Ascospores fusiform, oblong, sometimes navicular or allantoid, 12–22 × 3–5 μm, 0–3-septate, not constricted at the septa, hyaline, smooth.
Culture characteristics — Colonies on OA 90 mm diam after 1 wk, centre with some puffs of white aerial mycelium, periphery scarce aerial mycelium, salmon to saffron with slimy, peach spots, reverse similar; no exudate or soluble pigment produced. After 3 wk centre with some puffs of white aerial mycelium, saffron with slimy, peach, brick or dark brick spots.
Specimens examined. CANADA, Alberta, on barley, 1995, R. Clear, DAOM 250164; Manitoba, on grain, 2001, R. Clear, DAOM 250175, DAOM 250174, DAOM 250173, DAOM 250172, DAOM 250171, DAOM 250169, CPC 26001; Manitoba, Brandon, on Triticum aestivum, 2006, DAOM 250162, DAOM 250161; Saskatchewan, on grain, 1984, R. Clear, CPC 25993; Saskatchewan, on grain, 2001, R. Clear, DAOM 250168, DAOM 250167, DAOM 250166, DAOM 250165; Saskatchewan, on Triticum aestivum, 10 Jan. 2002, R. Clear, DAOM 250176; unknown substrate, 16 June 2005, R. Clear, DAOM 250163. – SWITZERLAND, Reckenholz, on maize kernels, 2005, B. Dorn (holotype CBS H-22139; living culture ex-type CBS 139951 = CPC 26019); 2006, B. Dorn, DAOM 250159, DAOM 250158; 2007, B. Dorn, DAOM 250155; unknown date, B. Dorn, CBS 122706, CBS 122707.
Notes — The M. seminicola clade is represented by 23 strains collected mainly in Canada and Switzerland. Most strains remained sterile. Conidiophores and conidia observed in CBS 139951 and CBS 122706 were very similar. The sexual morph was only observed in CBS 139951. Microdochium seminicola was phylogenetically closely related to M. albescens (Fig. 2). Nevertheless, M. albescens, the causal agent of leaf-scald disease of rice, is different from M. seminicola. Microdochium albescens has larger ascospores with more septa (14–30 × 3.5–7.5 μm, 1–5-septate), and the asexual morph has falcate conidia, 11–16 × 3.5–4.5 μm conidia that are 0–3-septate but usually 1-septate, (Parkinson et al. 1981) while M. seminicola has smaller ascospores with fewer septa (12–22 × 3–5 μm, up to 3-septate), and the asexual morph has larger conidia with more septa (23–54 μm long, up to 5-septate, usually 3-septate). Morphologically M. seminicola, which has been isolated from maize in Switzerland, also resembles M. maydis. However, conidia of M. maydis are smaller with more septa (20–46 × 3–4 μm, 3–9-septate). In addition, M. maydis was isolated from maize leaves whereas, although M. seminicola occurs on maize kernels, it is most often isolated from harvested grain, including oats, barley, and wheat grain, and rarely canola seed. Unfortunately, there are no cultures or molecular data available for M. maydis.
For decades, Canadian seed testing laboratories have been puzzled by the sporadic occurrence, sometimes at frequencies of 3–4 % within a seed lot, of this fast growing fungus, with white to pinkish colonies that superficially resemble those produced by some Fusarium species. However, the colonies often remain sterile on PDA, the medium used in most seed testing procedures. A few orange sporodochia are sometimes produced, but this sporulation disappears after one or two transfers, resulting in sterile, relatively fast-growing light orange colonies.
Microdochium stevensonii (Petr.) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811972
Basionym. Griphosphaerella stevensonii Petr., Ann. Mycol. 25: 209. 1927.
≡ Griphosphaeria stevensonii (Petr.) E. Müll. & Arx, Phytopathol. Z. 24: 355. 1955.
≡ Monographella stevensonii (Petr.) Arx, The genera of fungi sporulating in pure culture: 174. 1981.
Microdochium trichocladiopsis Hern.-Restr. & Crous, sp. nov. — MycoBank MB811876; Fig. 15
Fig. 15.
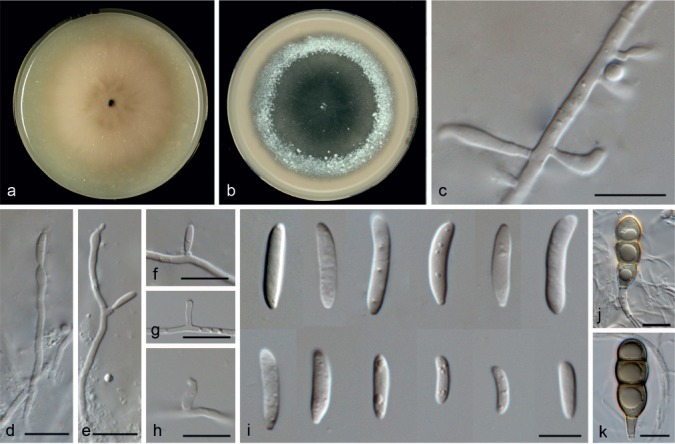
Microdochium trichocladiopsis (from ex-type, CBS 623.77). a. Colony on OA at 25 °C in 1 wk; b. colony on OA at 25 °C in 3 wk; c–h. conidiogenous cells; i. conidia; j, k. chlamydospores. — Scale bars: c–k = 10 μm.
Etymology. Trichocladiopsis, referring to the chlamydospores of this taxon that superficially resemble conidia of Trichocladium species.
Mycelium mostly immersed, hyphae hyaline, branched, smooth. Conidiogenous cells sparse, solitary, cylindrical to clavate, straight, often curved at the tip, hyaline, smooth, 4–37.5 × 2–3 μm. Conidia oblong, fusiform to obovoid, straight or curved, 6–18 × 2–3.5 μm, 0(–1)-septate, base usually flattened. Chlamydospores abundant, terminal, obovoid, pyriform to clavate, trichocladium-like, 1–3(–5)-septate, brown to dark brown, basal cells often paler, constricted at the septa, sometimes with a pale brown frill at the base.
Culture characteristics — Colonies on OA 80 mm diam after 1 wk, flat, lacking aerial mycelium, rosy buff, black near to the inoculum, margin diffuse, reverse similar. After 3 wk, centre with sparse to absent aerial mycelium, radially striate, dark grey olivaceous, periphery aerial mycelium floccose white, margin diffuse, saffron, reverse olivaceous grey with concentric rings of pale mouse grey.
Specimen examined. UNKNOWN COUNTRY, from rhizosphere of Triticum aestivum, unknown date, J.W.L. van Vuurde (holotype CBS H-22137; living culture ex-type CBS 623.77).
Notes — Isolate CBS 623.77 formed a clade with two isolates of M. tainanensis, CBS 269.76 and CBS 270.76. Both species are clearly distinguished morphologically. In M. tainanensis the conidia are lunate and smaller (10–15 × 2–3 μm), while M. trichocladiopsis produces oblong to fusiform and larger conidia (4–37.5 × 2–3 μm). Species in this clade (Fig. 2) are associated with roots and produce brown chlamydospores. Microdochium trichocladiopsis was isolated from the rhizosphere of Triticum aestivum and has chlamydospores with up to 5 septa, while both isolates of M. tainanense were isolated from roots of Saccharum officinarum, and have aseptate chlamydospores (De Hoog & Hermanides-Nijhof 1977).
Selenodriella cubensis Hern.-Restr. & Crous, sp. nov. — MycoBank MB811877; Fig. 16
Fig. 16.
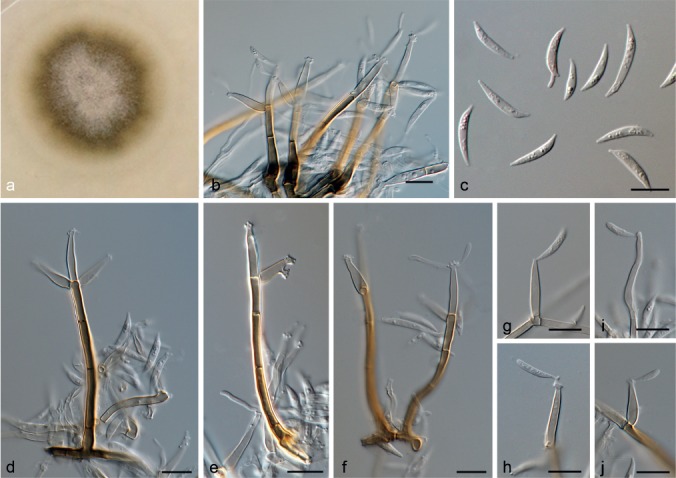
Selenodriella cubensis (from ex-type, CBS 683.96). a. Colony on OA at 25 °C in 1 wk; b, d–f. conidiophores; c. conidia; g–j. conidiogenous cells. — Scale bars: b–j = 10 μm.
Etymology. Named after the country where this fungus was collected, Cuba.
Mycelium immersed and superficial, hyphae hyaline to pale brown, branched, septate, smooth. Conidiophores erect, setiform, branched at the apex, brown at the base, becoming hyaline at the apex, 49–87 × 2.5–4 μm. Conidiogenous cells cylindrical to lageniform, sympodial, denticulate, terminal, in whorls at the apex or solitary on the mycelial hyphae, hyaline to subhyaline, 13–39 × 2–4 μm. Conidia lunate, asymmetrical, 10–20 × 2–3 μm, unicellular, hyaline, smooth-walled, guttulate. Chlamydospores not observed. Sexual morph unknown.
Culture characteristics — Colonies on OA 17–19 mm diam in 1 wk. Zonate, velvety to powdery, buff in the centre; sparse aerial mycelium, umber towards the periphery; margin diffuse.
Specimen examined. CUBA, unknown substrate, June 1996, R.F. Castañeda (holotype INIFAT C96/30; living culture ex-type CBS 683.96; CBS H-22143 dry culture).
Notes — The isolate CBS 683.96, formerly identified as Idriella tropicalis, is better accommodated in Selenodriella, since it produces setiform conidiophores, and conidiogenous cells and branches are disposed in whorls along the main axis of setiform conidiophores as in species of Selenodriella. It differs from Idriella, which shows conidiophores reduced to conidiogenous cells. The only available strain of Selenodriella cubensis clustered phylogenetically close to Selenodriella fertilis (CBS 772.83) in Microdochiaceae. Selenodriella fertilis, the type species of the genus, and S. cubensis, differs mainly in the arrangement of the conidiogenous cells. In S. fertilis they are arising in groups in the middle part of the conidiophores (Pirozynski & Hodges 1973), while in S. cubensis the conidiogenous cells are disposed at the apex of the conidiophores. Conidial morphology is slightly different in both species; in S. cubensis conidia are pointed at both ends, while in S. fertilis they are flat at the base and rounded at the apex.
Sordariomycetes, Xylariales, incertae sedis
Castanediella couratarii (C. Ram) Hern.-Restr. & Crous, comb. nov. — MycoBank MB812166; Fig. 17
Fig. 17.
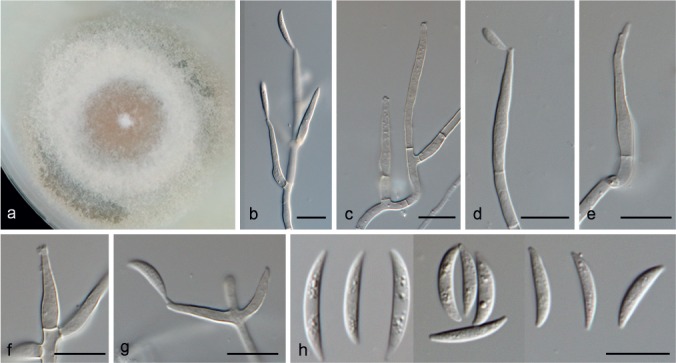
Castanediella couratarii (from ex-type, CBS 579.71). a. Colony on OA at 25 °C in 1 wk; b–g. conidiogenous cells; h. conidia. — Scale bars: b–h = 10 μm.
Basionym. Idriella couratarii C. Ram (as ‘couratorii’), Brotéria Ci. Nat. 39: 27. 1970.
Mycelium immersed and superficial, hyphae branched, septate, hyaline and brown. Conidiophores mostly branched, pale brown. Conidiogenous cells lageniform to cylindrical, solitary or in whorls, straight or flexuous, hyaline to pale brown, 10.5–37 × 2–3.5 μm. Conidia lunate, hyaline, 9.5–19 × 2–3 μm. Chlamydospores not observed.
Culture characteristics — Colonies on OA reaching 30 mm diam in 2 wk. Fluffy aerial mycelium, zonate, rosy vinaceous in the centre, white to the periphery, margin grey-sepia.
Specimen examined. BRAZIL, on dead wood, Aug. 1971, J.L. Bezerra (holotype IMUFPe 2222; living culture ex-type CBS 579.71 = ATCC 22642; CBS H-22141 dry).
Notes — Castanediella was recently introduced with C. acacia as type species (Crous et al. 2015). The main distinguishing character between Castanediella and Idriella is in their conidiophore morphology. Conidiophores in Castanediella are commonly branched, while in Idriella conidiophores are mostly reduced to conidiogenous cells. The two genera are also phylogenetically distinct (Fig. 1).
Idriellopsis Hern.-Restr. & Crous, gen. nov. — MycoBank MB811882
Etymology. In reference to its morphological similarity with the genus Idriella.
Type species. Idriellopsis uncinospora (R.F. Castañeda & W.B. Kendr.) Hern.-Restr. & Crous.
Mycelium immersed and superficial, hyphae septate, branched, smooth-walled, brown or pale brown. Conidiophores differentiated, unbranched inflated or globose at the apex, brown at the base, almost hyaline at the apex, smooth-walled. Conidiogenous cells polyblastic, terminal, integrated, with conspicuous denticles. Conidia falcate, curved, with one side straighter that the other, tapered at the base, rounded at the apex 0–1-septate, hyaline, smooth-walled. Chlamydospores not observed. Sexual morph unknown.
Idriellopsis uncinospora (R.F. Castañeda & W.B. Kendr.) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811883; Fig. 18
Fig. 18.
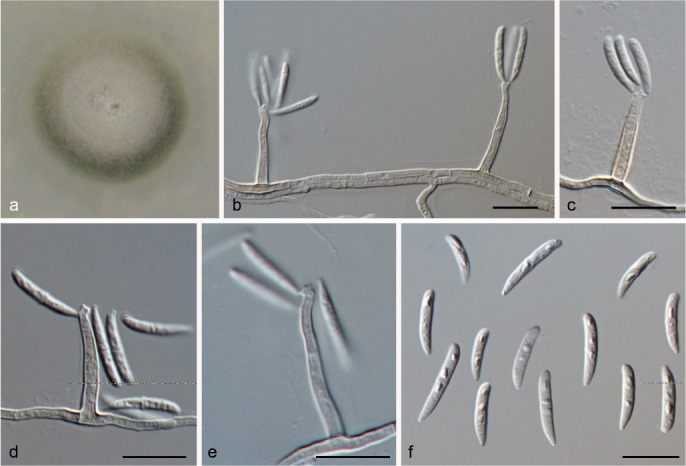
Idriellopsis uncinospora (from ex-type, CBS 575.92). a. Colony on OA at 25 °C in 1 wk; b–e. conidiogenous cells; f. conidia. — Scale bars: b–f = 10 μm.
Basionym. Idriella uncinospora R.F. Castañeda & W.B. Kendr., Univ. Waterloo Biol. Ser. 35: 68. 1991.
Description on natural substrate — Castañeda-Ruiz & Kendrick (1991).
Mycelium immersed and superficial, hyphae branched, septate, hyaline to pale brown. Conidiophores simple, pale brown at the base, subhyaline to hyaline at the apex, 0–1-sepate, 13–35 × 2–3.5 μm. Conidiogenous cells polyblastic, denticulate, cylindrical and inflated at the apex, 11–21 × 2–3.5 μm, pale brown, subhyaline to hyaline at the apex. Conidia subfalcate, curved, tapered at the base, rounded and curved at the apex, 0–1-septate, guttulate, smooth-wall, hyaline, 9–15 × 1.5–2 μm. Chlamydospores not observed.
Culture characteristics — Colonies on OA reaching 16–23 mm diam in 3 wk, flat, powdery, pale mouse grey, margin olivaceous or buff, diffuse.
Specimen examined. CUBA, Santiago de Las Vegas, on dead leaf, 18 Feb. 1991, R.F. Castañeda (holotype INIFAT C91/69; living culture ex-type CBS 575.92; CBS H-22145 dry).
Notes — Idriellopsis uncinospora is phylogenetically distant (Fig. 1) from Idriella s.str., although morphologically, it appears similar with pale brown conidiophores, denticulate conidiogenous cells and curved conidia. Nevertheless, the conidial morphology is slightly different, since in I. uncinospora conidia are 0–1-sepate and have truncate bases with obtuse and curved apices, whereas in Idriella s.str. conidia are non-septate and have acuminate bases and apices. Idriellopsis uncinospora is represented by one isolate (CBS 575.92), originally described growing on dead leaves from Cuba (Castañeda-Ruiz & Kendrick 1991).
Neoidriella Hern.-Restr. & Crous, gen. nov. — MycoBank MB811884
Etymology. In reference to its similarity with the genus Idriella.
Type species. Neoidriella desertorum (Nicot & Mouch.) Hern.-Restr. & Crous.
Mycelium immersed and superficial, hyphae hyaline to pale brown, branched, septate, smooth. Conidiophores mostly simple, pale brown. Conidiogenous cells sympodial, denticulate, terminal. Conidia unicellular, hyaline, cylindrical to obovoid, smooth-walled. Chlamydospores intercalary or terminal, pale brown. Sexual morph unknown.
Neoidriella desertorum (Nicot & Mouch.) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811885; Fig. 19
Fig. 19.
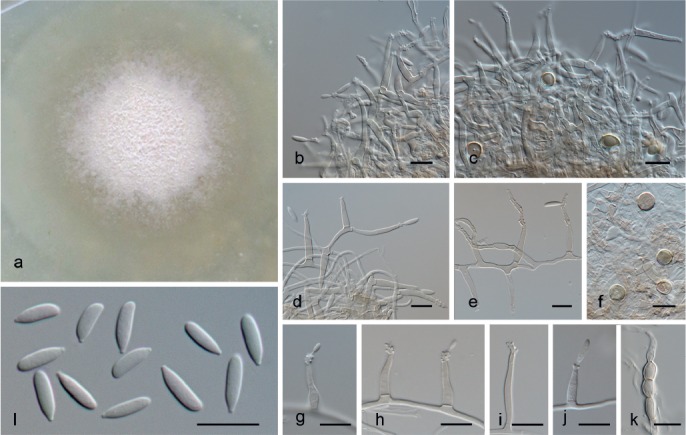
Neoidriella desertorum (from ex-type, CBS 985.72). a. Colony on OA at 25 °C in 1 wk; b–e, g–j. conidiogenous cells; f, k. chlamydospores; l. conidia. — Scale bars: b–l = 10 μm.
Basionym. Idriella desertorum Nicot & Mouch., Rev. Mycol. (Paris) 36: 192. 1972.
Mycelium immersed and superficial, hyphae hyaline to pale brown, branched, septate, smooth. Conidiophores simple or branched, pale brown. Conidiogenous cells cylindrical, wider at the base, sympodial, denticulate, terminal or lateral, straight or flexuous, 10.5–38 × 2–4 μm, with rachis, 4–21.5 × 1–3 μm. Conidia cylindrical to obovoid, 7–10 × 2–3 μm, unicellular, hyaline, smooth-walled, base tapered, apex rounded. Chlamydospores mostly globose, 5.5–9 μm diam, uni- or pluricellular, intercalary or terminal, pale brown. Sexual morph unknown.
Culture characteristics — Colonies on OA reaching 35–40 mm diam in 3 wk. Zonate, centre velvety to cottony, rosy buff; periphery glabrous, ocreous; margin entire.
Specimen examined. EGYPT, Western Desert, from desert soil, Nov. 1972, J. Mouchacca (holotype CBS H-7247; living culture ex-type CBS 985.72 = ATCC 26429 = IMI 171136 = LCP 2115).
Notes — The single available isolate of this species clustered on a separate branch in Xylariales, separated from the type species of the genus Idriella. The conidiophores in N. desertorum are mostly reduced to conidiogenous cells as in Idriella, but the conidial shape in N. desertorum is obovoid to clavate tapering at the base, different from Idriella that has lunate conidia. Furthermore, the conidiogenous cells develop a rachis with conspicuous denticles.
Paraidriella Hern.-Restr. & Crous, gen. nov. — MycoBank MB811886
Etymology. In reference to its similarity with the genus Idriella.
Type species. Paraidriella jambosae (R.F. Castañeda & W.B. Kendr.) Hern.-Restr. & Crous.
Mycelium immersed and superficial, hyphae hyaline to pale brown, branched, septate, smooth. Conidiophores pale brown, mostly reduced to conidiogenous cells. Conidiogenous cells cylindrical to lageniform, sympodial, denticulate, terminal. Conidia unicellular, hyaline, cylindrical to oblong, smooth-wall. Chlamydospores not observed. Sexual morph unknown.
Paraidriella jambosae (R.F. Castañeda & W.B. Kendr.) Hern.-Restr. & Crous, comb. nov. — MycoBank MB811887; Fig. 20
Fig. 20.
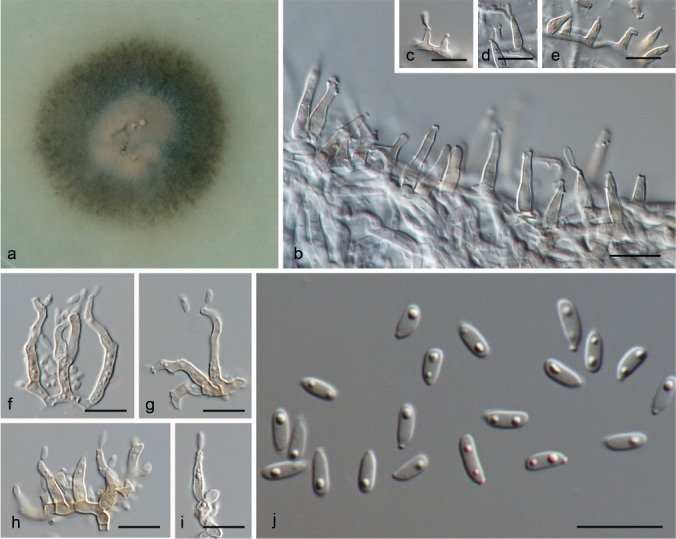
Paraidriella jambosae (from ex-type, CBS 374.90). a. Colony on OA at 25 °C in 1 wk; b–i. conidiogenous cells; j. conidia. — Scale bars: b–j = 10 μm.
Basionym. Idriella jambosae R.F. Castañeda & W.B. Kendr., Univ. Waterloo Biol. Ser. 35: 68. 1991.
Description on natural substrate — Castañeda-Ruiz & Kendrick (1991).
Mycelium immersed and superficial, hyphae hyaline to pale brown, branched, septate, smooth. Conidiophores pale brown, 0–1-sepate. Conidiogenous cells cylindrical to lageniform, inflated at the apex, sympodial, denticulate, terminal, straight or flexuous, 10–25 × 2–3 μm. Conidia unicellular, hyaline, cylindrical to oblong, asymmetrical, 4–5.5 × 1–1.5 μm, smooth-walled, 1–2 guttulate, base tapered, apex rounded. Chlamydospores not observed. Sexual morph unknown.
Culture characteristics — Colonies on OA reaching 22–28 mm diam in 3 wk, flat, velvety, zonate centre rosy buff, periphery isabelline, margin fimbriate.
Specimen examined. CUBA, San Juan y Martínez, Pinar del Rio, Cuchillas de San Simon, on fallen leaves of Syzygium jambos (= Jambosa vulgaris), 24 Mar. 1990, R.F. Castañeda (holotype CBS H-22146; living culture ex-type CBS 374.90)
Notes — Paraidriella jambosae is represented by the only available isolate of this species, and clustered on a separate branch in Xylariales. The only obvious morphological difference with Idriella lies in its conidia, which are cylindrical to oblong, tapered at the base and rounded at the apex in Paraidriella, differing from Idriella that has lunate conidia with pointed ends. However, phylogenetically both genera are clearly distinct.
DISCUSSION
Monographella for many years was considered the sexual morph of Microdochium. Nevertheless, with the implementation of ‘one fungus one name’ nomenclature, we propose to retain Microdochium as genus name. Microdochium and Monographella were described in the same journal volume in Annales Mycologici in 1924. Nevertheless, Microdochium has more species, is more commonly encountered, and the name is more frequently used in literature.
Microdochium and Idriella are morphologically similar, as well as phylogenetically related as shown in the present analyses (Fig. 1). Previous studies connected Idriella with Hymenoscyphus in Helotiales (Kimbrough & Atkinson 1972) and Microdochium with Amphisphaeriaceae (Parkinson et al. 1981, Samuels & Hallet 1983, Von Arx 1984, Jaklitsch & Voglmayr 2012). Amphisphaeriaceae is a large heterogeneous family which possesses pestalotiopsis-like asexual morphs characterised by holoblastic conidiogenous cells that produce septate, brown or hyaline conidia with appendages at both ends (Tanaka et al. 2011, Maharachchikumbura et al. 2014). Nevertheless, in our phylogenetic tree, Microdochium and Idriella formed a separate clade in Xylariales distinct from other families (Fig. 1). Based on the results of our phylogenetic analyses Microdochium, Idriella and Selenodriella correspond to a new family introduced here as Microdochiaceae. This new family is characterised by asexual morphs that produce polyblastic, sympodial or annellidic conidiogenous cells with hyaline conidia without appendages and sexual morphs that are monographella-like.
Species of Microdochium and Idriella are phytopathogenic and saprobic, differentiated morphologically mainly by the pigmentation of their conidiogenous cells, which are hyaline in Microdochium and pale brown in Idriella. The conidial shape seems to be another taxonomic important feature, while in Idriella the conidia are lunate with pointed ends, in Microdochium the conidial shape is more variable from cylindrical, fusoid or oblong, to lunate, straight or curved, with truncate bases and apices mainly rounded. The formation of chlamydospores was observed in both genera, but not seen in all Microdochium species. Nevertheless, morphological characters used to delimit species in Microdochium including conidiomatal structure, conidiogenous cells and conidia were frequently found degenerated in cultures. For example in M. phragmites, the type species of the genus represented by two strains with morphological differences; one of them shows denticulate conidiogenous cells and the other one produces annellidic conidiogenous cells. Nevertheless they were genetically similar. Sporodochia and ascomata commonly described from natural substrates in cultures were poorly developed or absent in some cultures. On the other hand sexual and asexual connections as in M. lycopodinum were based only on molecular data. Furthermore, some species are morphologically very similar and difficult to distinguish based on literature, as in the case of M. neoqueenslandicum and M. colombiense. Species boundaries drawn in the present study were based primarily in statistically well-supported branches in multi-locus phylogenies. By combining DNA sequences with morphological analyses, we were able to delimit and propose six new species among the fungi formerly recognised in Microdochium, namely Microdochium citrinidiscum, M. colombiense, M. fisheri, M. neoqueenslandicum, M. seminicola and M. trichocladiopsis, which differed from other species in the genus (Table 2). Since Monographella is treated as synonym of Microdochium, we furthermore propose six new combinations in Microdochium as M. albescens, M. consociatum, M. fusariisporum, M. maydis, M. opuntiae and M. stevensonii.
Table 2.
Overview of morphological characters of Microdochium spp.
| Asexual morph |
Sexual morph |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conidia |
Conidiogenous cells |
Chlamydospores |
Perithecia |
Asci |
Ascospores |
|||||
| Taxa | Shape | Size (μm) | # septa | Type - Shape | Size (μm) | Type | Size/Diameter (μm) | Size (μm) | Size (μm) | # septa |
| M. albescens | falcate, slightly to strong curved, apex pointed | 11–16 × 3.5–4.5 | 0–1(–3) | percurrent, subcylindrical, doliiform to obpyriform | 6–15 × 1.5–4 | not observed | 150–180 × 90–120 | 40–85 × 8–12 | 14–23 × 3.5–4.5 | 1–3(–5) |
| M. bolleyi | crescent | 5.5–8.5 × 1.6–2.2 | 0 | sympodial, cylindrical or ampulliform, with rachides | 2–4.5 × 2–3.5 | present, multicellular | not reported | not reported | not reported | not reported |
| M. caespitosum | falcate, pointed | 25–30 × 1.5–2 | 1 | sympodial, ampulliform | 7.5–15 × 2.5–5 | not reported | not reported | not reported | not reported | not reported |
| M. citrinidiscum | cylindrical, clavate, obovoid | 7–31 × 2–3 | 0–3 | sympodial, denticulate, cylindrical | 11–29 × 1.5–2 | not observed | not reported | not reported | not reported | not reported |
| M. colombiense | lunate, fusiform, allantoid or reniform, straight or curved | 5–8 × 1.5–3 | 0(–1) | polyblastic, ampulliform, with per-current proliferations, or cylindrical | 5–13 × 2.5–3.5 | not observed | not reported | not reported | not reported | not reported |
| M. consociata | not described | present – not described | present – not described | present – not described | present – not described | present – not described | 110–300 | 90–120 × 21–25 | 32–38 × 8–11 | 3–6 |
| M. fisheri | obovoid, subpyriform, to clavate, fusiform | 7–12 × 3–4 | 0–1 | sympodial, denticulate, cylindrical | 19–60 × 1.5–2 | not observed | not reported | not reported | not reported | not reported |
| M. fusariisporium | not reported | not reported | not reported | not reported | not reported | not reported | 165–220 × 137–165 | 45–65 × 8–9 | 20–32 × 3–3.5 | 1–3 |
| M. griseum | falcate, pointed at both ends | 20–30 × 2–2.5 | 0 | sympodial, apical, ampulliform, up to 5 denticles | < 30 × 1–4.5 | not reported | not reported | not reported | not reported | not reported |
| M. intermedium | fusiform, falcate | 8–15 × 3–4.5 | 1–2 | sympodial, cylindrical or ampulliform with short denticulate rachides | 10–20 × 3–4 | not reported | not reported | not reported | not reported | not reported |
| M. lycopodinum | fusiform or with one side straighter than the other, lunate | 8–15 × 2.5–3.5 | 0–1 | ampulliform to lageniform, sub-cylindrical, percurrent proliferations | 4–12 × 2.5–3.5 | not observed | 80–190 | 37–66 × 5–7.5 | 9–24 × 2–3.5 | 1(–2–3) |
| M. majus | falcate, slightly to strong curved, apex pointed, base wedge-shaped | 19–37 × 3.5–4.5 | (1–)7 | percurrent, apical, subcylindrical, doliiform to obpyriform | 6–15 × 2.2–4 | not reported | 300 × 170 | 50–70 × 7–9 | 9.5–17 × 3–4.5 | 1–3 |
| M. maydis | cylindrical to slightly clavate, apex obtuse, base narrowed, mostly curved | 20–46 × 3–4 | 3–9 | percurrent, apical, doliiform, ampulliform to obpyriform | 15–20 × 10 | not reported | 200–250 × 100–200 | 80–90 × 10–12 | 18–25 × 3.5–5 | 1–3 |
| M. neoqueenslandicum | lunate, allantoid, curved, with one side straighter than the other | 4–9 × 1.5–3 | 0(–1) | ampulliform, lageniform to subcylindrical, polyblastic, percurrent proliferations | 4.5–10 × 2–3.5 | not observed | not reported | not reported | not reported | not reported |
| M. nivale | falcate, slightly to strong curved, apex pointed, base wedge-shaped, base obtuse to round | 5–36 × 2–4.5 | 3(–1–7) | percurrent, apical, subcylindrical, doliiform to obpyriform | 6–15 × 2.2–4 | not reported | 300 × 170 | 50–70 × 7–9 | 10–17 × 3.5–4.5 | 1(–3) |
| M. opuntiae | not reported | not reported | not reported | not reported | not reported | not reported | 100–112 | > 60 × 8–9 | 20–22 × 3.5 | 1 |
| M. palmicola | filiform, straight to slighlty flexuous, apex rounded | 7–16 × 1 | 0 | sympodial, apical, ampulliform to lageniform | 6–13 × 2.5–5(–7) | not reported | not reported | not reported | not reported | not reported |
| M. paspali | falcate, apex pointed | 7–20.5 × 2.5–4.5 | 0–3 | percurrent, ampuliform, lageniform to cylindrical | 6.5–15.5 × 2.5–4 | not reported | not reported | not reported | not reported | not reported |
| M. passiflorae | falcate | 28–50 × 3–3.5 | 1–6 | sympodial, apical, cylindrical to doliiform | 10–15 × 3–4 | not reported | 200–250 | 57–120 × 9–11 | 15–25 × 4–5 | 1–3 |
| M. phragmitis | narrowly ellipsoid-fusiform, slightly curved, somewhat falcate apex obtuse to subacute, tapered, base somewhat obconically | 10–16 × 2–3.5 | 0–1 | sympodial, apical, ampulliform to lageniform | 5–12(–30) × 2.5–3 | not observed | not reported | not reported | not reported | not reported |
| M. punctum | fusiform, straight, apex rounded to subacute | 20–30 × 3–5 | 1 | subcylindrical, ampulliform, conical to geniculate-sinuous | 5–8(–15) × 2–3 | not reported | not reported | not reported | not reported | not reported |
| M. queenslandicum | lunate | 7.5–11 × 1.8–2 | 0 | sympodial, apical, ampulliform | 4–7 × 2–3 | not reported | not reported | not reported | not reported | not reported |
| M. seminicola | cylindrical to fusiform, straight or curved | 19–54 × 3–4.5 | (0–)3(–5) | ampulliform to lageniform, with percurrent proliferations | 7–9.5 × 3–4 | not observed | 110–149 | 41–66 × 7.6–11 | 12–22 × 3–4.5 | 0–3 |
| M. sorghi | filiform, narrowly acicular fusiform, obclavate | 20–90 × 1.5–4.5 | 1–7(–10) | sympodial, occasionally percurrent. Ovoid, ampulliform to obclavate | 5–13 × 3–4 | not reported | not reported | not reported | not reported | not reported |
| M. stevensonii | not reported | not reported | not reported | not reported | not reported | not reported | 150–260 | 45–65 × 11–13 | 14–21 × 5–6.5 | 1–2 |
| M. stoveri | cylindrical to fusiform, often curved, apex rounded | 13–39 × 2–3 | 0–2 | sympodial, apical, cylindrical | 6.5–15 × 2.5–3.5 | not reported | 120 × 90 | 75–115 × 18–28 | 23–30 × 5.5–6.5 | 3–4 |
| M. tainanense | lunate | 10–15 × 2–3 | 0–1 | sympodial, apical, cylindrical or ampulliform with conspicuous rhachides | 3–10 × 1–3 | not observed | not reported | not reported | not reported | not reported |
| M. trichocladiopsis | oblong, fusiform to obovoid, straight or curved | 6–18 × 2–3.5 | 0(–1) | cylindrical to clavate, straight often curved at the tip | 4–37 × 2–3 | present, trichocladium like | not reported | not reported | not reported | not reported |
| M. triticicola | fusiform, straight | 5–14 × 2.5–4 | (0–)1 | sympodial, apical, ampulliform, lageniform to cylindrical | 6.5–35 × 2.5–3.5 | not reported | not reported | not reported | not reported | not reported |
For an accurate species identification of Microdochium species, a molecular analysis is required. The four gene regions used in this study were chosen based on their previous use in molecular studies (Jaklitsch & Voglmayr 2012, Jewell & Hsiang 2013, Zhang et al. 2015). LSU was only usefull for generic placement, since it was not able to separate M. seminicola, M. albescens, M. majus and M. nivale. Although phylogenetic analyses of the individual gene regions of ITS, BTUB and RPB2 (results not shown) were able to resolve 14 species in Microdochium with varying statistical support they proved to be suitable barcoding markers for species identification. The phylogeny based on BTUB showed longer distances between species and higher support values. This locus was the most informative of the three gene regions studied, which is in agreement with previous studies in other xylariaceous genera (Hsieh et al. 2005, Læssøe et al. 2013). After this revision Microdochium s.str. includes 29 species, of which the main morphological characters are summarised in Table 2. Some previously published species of Microdochium and Idriella clustered outside the Microdochiaceae. These include the isolate CBS 493.70, which was originally recognised as Microdochium gracile, and is shown here to represent Paramicrodochium gracile gen. et comb. nov. (Sordariomycetes incertae sedis), and the isolate CBS 857.72, which was originally included as Microdochium tripsaci, and shown here to represent Ephelis tripsaci comb. nov. (Clavicipitaceae, Hypocreales), and CBS 740.83, CBS 741.83 and CBS 742.83, which were originally described as Microdochium fusarioides, and are shown here to represent Microdochiella fusarioides gen. et comb. nov. (Orbiliales) (Fig. 1). In addition we propose three new genera based on species formerly described as Idriella, but shown to be phylogenetically distinct genera introduced as Idriellopsis to accommodate Idriella uncinospora (CBS 575.92); Neoidriella to accommodate Idriella desertorum (CBS 985.72); and Paraidriella to accommodate Idriella jambosae (CBS 374.90). Furthermore, one new species is proposed in Selenodriella for S. cubensis (former identified as Idriella tropicalis) and a new combination Castanediella couratarii to accommodate Idriella couratarii CBS 579.71 was introduced.
For delineating those new idriella-like genera, besides phylogenetic differences, slight morphological differences were observed. Idriella is defined by having conidiophores reduced to pale brown, denticulate conidiogenous cells, with lunate, non-septate conidia, pointed at both ends, and dark chlamydospores. Idriella-like genera can be separated based on the branching pattern of their conidiophores and conidial shape and septation. Castanediella has branched conidiophores, conidiogenous cells with scars instead of denticles, and filiform, 0–1-septate conidia (Crous et al. 2015). Idriellopsis has conidiophores reduced to conidiogenous cells, falcate, curved and rounded at the apex, 0–1-septate conidia. Neoidriella has conidiophores that are mostly reduced to a conidiogenous cells, with unicellular, cylindrical to oblong, tapered bases and rounded apices and chlamydospores. Paraidriella has conidiophores that are mostly reduced to conidiogenous cells, with cylindrical to oblong, asymmetrical conidia.
Acknowledgments
We thank Keith A. Seifert, Randy Clear and Lawrence Thomson for providing strains and for valuable information of M. seminicola, and the technical staff of the CBS, Arien van Iperen (cultures) and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Braun U. 1995. A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Vol. 1 IHW Verlag, Eching. [Google Scholar]
- Castañeda-Ruiz RF, Kendrick B. 1991. Ninety-nine conidial fungi from Cuba and three from Canada. University of Waterloo Biology Series 35: 62–69. [Google Scholar]
- Chen J, Xu LL, Liu B, et al. 2007. Taxonomy of Dactylella complex and Vermispora. III. A new genus Brachyphoris and revision of Vermispora. Fungal Diversity 26: 127–142. [Google Scholar]
- Crous PW, Braun U, Hunter GC, et al. 2013. Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, et al. 2009a. Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. 2009b. Fungal biodiversity. CBS Laboratory Manuals Series 1. CBS-KNAW Fungal Biodiversity Centre Utrecht, The Netherlands. [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2015. Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, et al. 2006. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- De Hoog GS, Hermanides-Nijhof EJ. 1977. Survey of the black yeasts and allied fungi. Studies in Mycology 15: 178–222. [Google Scholar]
- Glynn NC, Hare MC, Parry DW, et al. 2005. Phylogenetic analysis of EF-1 alpha gene sequences from isolates of Microdochium nivale leads to elevation of varieties majus and nivale to species status. Mycological Research 109: 872–880. [DOI] [PubMed] [Google Scholar]
- Harris DC. 1985. Microdochium fusarioides sp. nov. from oospores of Phytophthora syringae. Transactions of the British Mycological Society 84: 358–361. [Google Scholar]
- Hock J, Dittrich U, Renfro BL, et al. 1992. Sequential development of pathogens in the maize tar-spot disease complex. Mycopathologia 117: 157–161. [Google Scholar]
- Hong SK, Kim WG, Choi HW, et al. 2008. Identification of Microdochium bolleyi associated with basal rot of creeping bent grass in Korea. Mycobiology 36: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HM, Ju YM, Rogers JD. 2005. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 97: 844–865. [DOI] [PubMed] [Google Scholar]
- Hyde KD, Jones EBG. 1986. Marine fungi from Seychelles. II. Lanspora coronata gen. et sp. nov. from driftwood. Canadian Journal of Botany 64: 1581–1585. [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2012. Phylogenetic relationships of five genera of Xylariales and Rosasphaeria gen. nov. (Hypocreales). Fungal Diversity 52: 75–98. [Google Scholar]
- Jewell LE, Hsiang T. 2013. Multigene differences between Microdochium nivale and Microdochium majus. Botany 91: 99–106. [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software v. 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough JW, Atkinson M. 1972. Cultural features and imperfect stage of Hymenoscyphus caudatus. American Journal of Botany 59: 165–171. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE. 1995. Fungi on Juncus roemerianus. 3. New Ascomycetes. Botanica Marina 38: 175–186. [Google Scholar]
- Kuldau GA, Liu J-S, White JF, Jr, et al. 1997. Molecular systematics of Calvicipitaceae supporting monophyly of genus Epichloe and form genus Ephelis. Mycologia 89: 431–441. [Google Scholar]
- Læssøe T, Srikitikulchai P, Luangsa-Ard JJD, et al. 2013. Theissenia reconsidered, including molecular phylogeny of the type species T. pyrenocrata and a new genus Durotheca (Xylariaceae, Ascomycota). IMA Fungus 4: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hyde KD, Jeewon R, et al. 2005. Phylogenetics and evolution of nematode-trapping fungi (Orbiliales) estimated from nuclear and protein coding genes. Mycologia 97: 1034–1046. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Wehlen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Maharachchikumbura SSN, Hyde KD, Groenewald JZ, et al. 2014. Pestalotiopsis revisited. Studies in Mycology 79: 121–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima T. 1971. Microfungi of the Solomon Islands & Papua - New Guinea. Kobe, Japan. [Google Scholar]
- Matsushima T. 1975. Icones Microfungorum: a Matsushima lectorum. Kobe, Japan. [Google Scholar]
- Mouchacca J, Samson RA. 1973. Deux nouvelles espèces du genre Microdochium Sydow. Revue de Mycologie 37: 267–275. [Google Scholar]
- Müller E, Samuels GJ. 1984. Monographella maydis sp. nov. and its connection to the tar-spot disease of Zea mays. Nova Hedwigia 40: 112–120. [Google Scholar]
- Nelson PE, Wilhelm S. 1956. An undescribed fungus causing a root rot of strawberry. Mycologia 48: 547–551. [Google Scholar]
- Nicot J, Mouchacca J. 1972. Une nouvelle espece de genre Idriella. Reveu de Mycologie 36: 185–193. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2.2. Uppsala: distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Parkinson VO, Sivanesan A, Booth C. 1981. The perfect state of the rice leaf-scald fungus and the taxonomy of both the perfect and imperfect states. Transactions of the British Mycological Society 76: 59–69. [Google Scholar]
- Pirozynski KA, Hodges CS., Jr 1973. New Hyphomycetes from South Carolina. Canadian Journal of Botany 51: 151–173. [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Kew, Surrey, UK: CMI and British Mycological Society. [Google Scholar]
- Rodrigues KF, Samuels GJ. 1992. Idriella species endophytic in palms. Mycotaxon 43: 271–276. [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels GJ, Hallett IC. 1983. Microdochium stoveri and Monographella stoveri, new combinations for Fusarium stoveri and Micronectriella stoveri. Transactions of the British Mycological Society 81: 473–483. [Google Scholar]
- Seifert KA, Morgan-Jones G, Gams W, et al. 2011. The Genera of Hyphomycetes. CBS Fungal Biodiversity Series 9: 1–997. Utrecht, the Netherlands: CBS-KNAW Fungal Biodiversity Centre. [Google Scholar]
- Sutton BC, Pirozynski KA, Deighton FC. 1972. Microdochium Syd. Canadian Journal of Botany 50: 1899–1907. [Google Scholar]
- Swofford DL. 2003. PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Sydow H. 1924. Sydow, Mycotheca germanic. Fasc. XLII-XLV (No. 2051–2250). Annales Mycologici 22: 257–268. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Endo M, Hirayama K, et al. 2011. Phylogeny of Discosia and Seimatosporium, and introduction of Adisciso and Immersidiscosia genera nova. Persoonia 26: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid generic identification and mapping enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Arx JA. 1981. Notes on Microdochium and Idriella. Sydowia 34: 30–38. [Google Scholar]
- Von Arx JA. 1984. Notes on Monographella and Microdochium. Transactions of the British Mycological Society 83: 373–374. [Google Scholar]
- Von Arx JA. 1987. Plant pathogenic fungi. Nova Hedwigia 87: 1–288. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DA, Sninsky JJ, et al. (eds), PCR Protocols: a guide to methods and applications: 315–322. Academic Press, Inc., San Diego. [Google Scholar]
- White JF, Jr, Bacon ChW, Hywel-Jones NL, et al. 2003. ClavicipitaIean fungi. Evolutionary biology, chemistry, biocontrol, and cultural impacts. Edited by Cook College-Rutgers University; New Brunswick, New Jersey. [Google Scholar]
- Wu HX, Schoch CL, Boonmee S, et al. 2011. A reappraisal of Microthyriaceae. Fungal Diversity 51: 189–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZF, Qiao M, Zhang Y, et al. 2011. Pseudotripoconidium, a new anamorph genus connected to Orbilia. Mycologia 103: 164–173. [DOI] [PubMed] [Google Scholar]
- Zhang W, Nan Z, Tian P, et al. 2015. Microdochium paspali, a new species causing seashore paspalum disease in southern China. Mycologia 107: 80–89. [DOI] [PubMed] [Google Scholar]


