Figure 1.
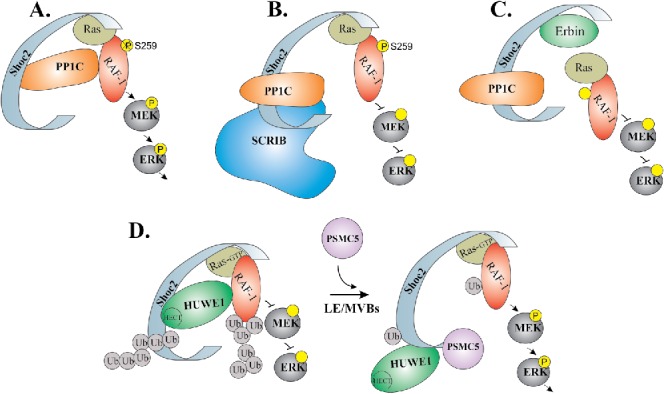
Schematic model depicting how ERK1/2 activity is transduced through the Shoc2 scaffold. Shoc2 routes Ras-Raf signals of the ERK1/2 pathway. (A) In the complex with Shoc2, PP1c dephosphorylates inhibitory Serine 259 of Raf-1 to facilitate signal transmission. (B) SCRIB competes with Shoc2 for PP1 binding thereby reducing ERK1/2 signaling. (C) Erbin disrupts the formation of Shoc2/Ras/Raf1 complexes to inhibit Shoc2-ERK1/2 signals. (D) Activation of the ERK1/2 pathway leads to an allosteric reversible ubiquitination of Shoc2 and, subsequently of Raf-1, by the E3 ligase HUWE1. HUWE1-mediated ubiquitination of Raf-1 fine-tunes its activity. In addition, ubiquitination triggers accumulation of Shoc2 complexes on the late endosomes/multi-vesicular bodies (LE/MVBs). On endosomes, Shoc2 complexes undergo remodeling by AAA+ ATPase PSMC5. The mechanoenzyme activity of this ATPases is utilized to reduce ubiquitination of Shoc2 and Raf-1 and, possibly, to reactivate the complex.
