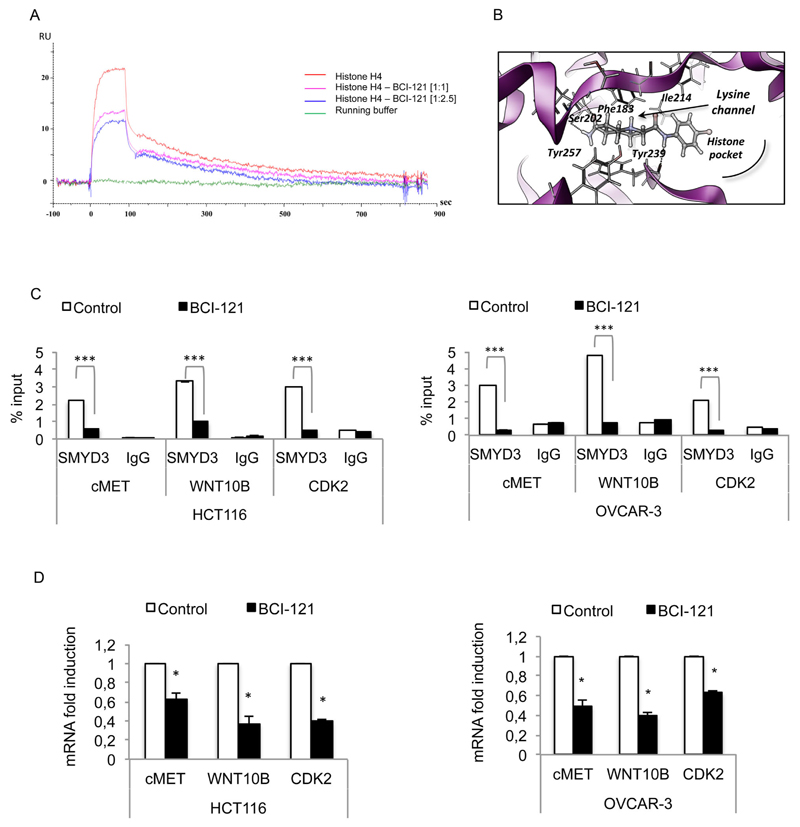
Fig. 9.
BCI-121 competes with target histones for SMYD3 binding in vitro and in cell line models. (A) Sensorgrams of H4 histone binding to SMYD3 in the presence and in the absence of BCI-121. (B) Predicted binding mode of BCI-121 to the histone binding site. (C) SMYD3 binding to the promoter of its target genes is abolished by the presence of BCI-121 in cancer cells. ChIP was performed in HCT116 and OVCAR-3 cells treated or not with BCI-121 (100 μM) for 72 h. Cells were cross-linked and immunoprecipitated with anti-SMYD3 and anti-IgG antibodies. The precipitated DNA was subjected to real-time PCR with specific primers, which amplify SMYD3 binding site elements of human target gene promoters (cMET, WNT10B, and CDK2). IgGs were used as an immunoprecipitation control. (D) Transcriptional activation of target genes correlates with impaired binding of SMYD3. β-actin was used for real-time PCR data normalization. Statistical analysis was performed using Student’s t-tail test; *P < 0.05 was considered statistically significant.