Significance
One of the oldest questions in human evolutionary studies is: why do Neandertals look different from present-day and ancient modern humans? This question can be addressed at different levels, but a critical component of a complete answer is understanding the developmental basis of adult differences. We now know that many skull differences are present by the time of birth. We know less, however, about the developmental basis of differences in the rest of the body. By studying the two most complete Neandertal neonates, we were able to establish that, as for the skull, many differences in body form are present by the time of birth. Neandertals largely look like Neandertals, regardless of age.
Keywords: body proportions, climatic adaptation, Homo neanderthalensis, infracranial, ontogeny
Abstract
Neandertal and modern human adults differ in skeletal features of the cranium and postcranium, and it is clear that many of the cranial differences—although not all of them—are already present at the time of birth. We know less, however, about the developmental origins of the postcranial differences. Here, we address this deficiency with morphometric analyses of the postcrania of the two most complete Neandertal neonates—Mezmaiskaya 1 (from Russia) and Le Moustier 2 (from France)—and a recent human sample. We find that neonatal Neandertals already appear to possess the wide body, long pubis, and robust long bones of adult Neandertals. Taken together, current evidence indicates that skeletal differences between Neandertals and modern humans are largely established by the time of birth.
It is well established that Neandertal and modern human adults differ in skeletal features of the cranium and postcranium (1–7). Furthermore, it is clear from multiple morphometric studies of the cranium (8–13) that many of the differences in cranial form—although not all of them—are already present around the time of birth. However, there have been only a few morphometric analyses of postcranial form in Neandertals < 1 y of age (11, 14, 15), although we know more about Neandertal children ∼2 y of age or older (16–20).
We would like to know if postcranial diversification followed a similar developmental path to cranial diversification. Additionally, knowing which Neandertal characteristics are present early in development has implications for determining whether postcranial differences between Neandertals and modern humans have evolutionary (e.g., genetic drift, natural selection) or lifetime behavioral (e.g., activity levels, gait patterns) causes (21). Newborn individuals are particularly important in this regard because their skeletons have been subject to only in utero mechanical loading, so it is not possible for their skeletons to have been influenced by locomotion or other habitual activities of adults or older subadults.
Here, we study the postcranial skeletons of the two most complete Neandertal neonates—Mezmaiskaya 1 (from Russia) and Le Moustier 2 (from France)—to quantify the extent to which Neandertal postcranial features are present around the time of birth. We base our analyses on 11 linear measurements (Table S1) of the Neandertals (Table S2) and a recent human sample of African Americans and European Americans (Tables S3 and S4).
Table S1.
Measurement descriptions
| Measurement | Basic definition | Source |
| Humerus length | Maximum length of humeral diaphysis | Fazekas and Kósa measurement 25a (41) |
| Humerus distal width | Maximum width of distal end of humeral diaphysis | Fazekas and Kósa measurement 25b (41) |
| Radius length | Maximum length of radial diaphysis | Fazekas and Kósa measurement 27a (41) |
| Radius distal width | Maximum width of distal end of radial diaphysis | This study |
| Ilium length | Maximum distance between anterior- and posterior-superior iliac spines of ilium | Fazekas and Kósa measurement 22a (41) |
| Ilium width | Maximum distance between middle of curvature of iliac crest and middle of curvature of iliac portion of acetabulum | Fazekas and Kósa measurement 22b (41) |
| Pubis length | Maximum distance between most ventral part of symphyseal and acetabular portions of pubis | Fazekas and Kósa measurement 24a (41) |
| Femur length | Maximum length of femoral diaphysis | Fazekas and Kósa measurement 28a (41) |
| Femur distal width | Maximum width of distal end of femoral diaphysis | Fazekas and Kósa measurement 28b (41) |
| Tibia length | Maximum length of tibial diaphysis | Fazekas and Kósa measurement 29a (41) |
| Tibia proximal width | Maximum width of proximal end of tibial diaphysis | This study |
Table S2.
Neandertal measurements
| Measurement | Mezmaiskaya 1 | Le Moustier 2 | ||||
| Left | Right | Value* | Left | Right | Value* | |
| Humerus length | x | — | 68 | — | x | 64 |
| Humerus distal width | x | x | 18 | x | — | 17 |
| Radius length | x | — | 52 | x | — | 54 |
| Radius distal width | x | — | 11 | x | — | 10 |
| Ilium length | — | x | 38 | x | — | 35 |
| Ilium width | — | x | 32 | x | — | 31 |
| Pubis length | x | x | 18 | — | — | — |
| Femur length | x | x | 78 | — | x | 72 |
| Femur distal width | x | x | 22 | x | — | 21 |
| Tibia length | x | x | 67 | — | — | — |
| Tibia proximal width | x | x | 17 | x | — | 15 |
Measurements defined in Table S1. An “x” indicates that the preservation was such that the side could be measured.
Average of the left and right sides when both sides could be measured; all measurements rounded to the nearest millimeter.
Table S3.
Recent human sample
| Group | Female | Male | Total |
| African American | 23 | 17 | 40 |
| European American | 13 | 15 | 28 |
| Total | 36 | 32 | 68 |
Table S4.
Summary statistics for the recent human sample
| Measurement | Sample size | Mean | SD |
| Humerus length | 68 | 68.2 | 7.0 |
| Humerus distal width | 68 | 17.1 | 2.2 |
| Radius length | 67 | 54.7 | 5.0 |
| Radius distal width | 67 | 9.3 | 1.4 |
| Ilium length | 66 | 34.7 | 4.4 |
| Ilium width | 66 | 31.1 | 3.7 |
| Pubis length | 49 | 16.3 | 2.5 |
| Femur length | 67 | 79.1 | 9.1 |
| Femur distal width | 67 | 20.2 | 3.1 |
| Tibia length | 66 | 68.8 | 7.4 |
| Tibia proximal width | 67 | 16.0 | 2.4 |
Measurements defined in Table S1.
Results
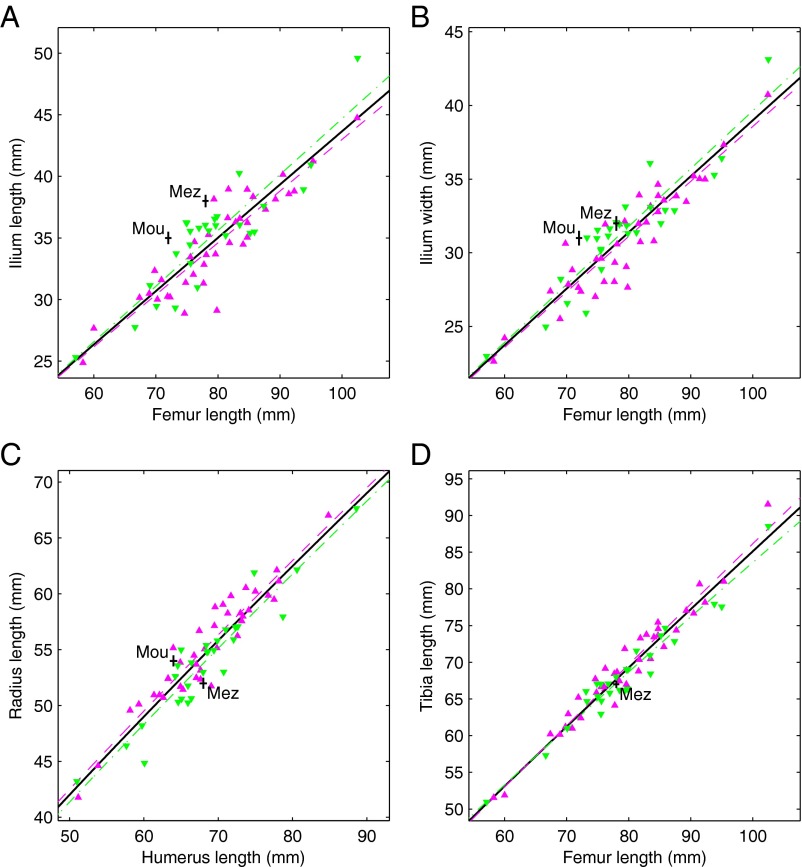
Adult European Americans and African Americans differ, on average, in body proportions, with European Americans having a wider trunk relative to limb length and relatively shorter distal limb segments (22, 23). These contrasts are much the same, although less extreme, as those between high- and low-latitude human groups (24, 25), and result from the geographic ancestries of European Americans and African Americans [Bergmann’s (26) and Allen’s (27) “rules”]. Similar proportional differences are already detectable in our fetal/infant sample (Fig. 1), which is consistent with other studies of subadult body proportions (28, 29). European Americans tend to have a wider and a longer ilium relative to femur length (Fig. 1 A and B), a shorter radius relative to humerus length (Fig. 1C), and a shorter tibia relative to femur length (Fig. 1D). Consistent with the “cold-adapted” body proportions of adult Neandertals (24, 30, 31), both Mezmaiskaya 1 and Le Moustier 2 have a very large ilium relative to femur length (Fig. 1 A and B), and Mezmaiskaya 1 has short distal-to-proximal limb lengths (Fig. 1 C and D; the results for tibia–femur proportions are less conclusive than for radius–humerus proportions because, although Mezmaiskaya 1 plots just below the European-American curve, the curves are minimally separated in this part of the graph). However, unexpectedly, Le Moustier 2 has a long radius relative to humerus length (Fig. 1C).
Fig. 1.
Body proportions. (A) Ilium length vs. femur length; (B) ilium width vs. femur length; (C) radius length vs. humerus length; and (D) tibia length vs. femur length (Table S1 includes measurement descriptions). The purple triangles are recent African Americans, the green inverted triangles are recent European Americans, and the black plus signs are Neandertals (Mez, Mezmaiskaya 1; Mou, Le Moustier 2). The plus-sign horizontal and vertical dimensions are ±0.5 mm. The purple dashed curve is the relationship for the African Americans, the green dash-dot curve is the relationship for the European Americans, and the black solid curve is the relationship for all recent humans (Table S6 includes fit statistics).
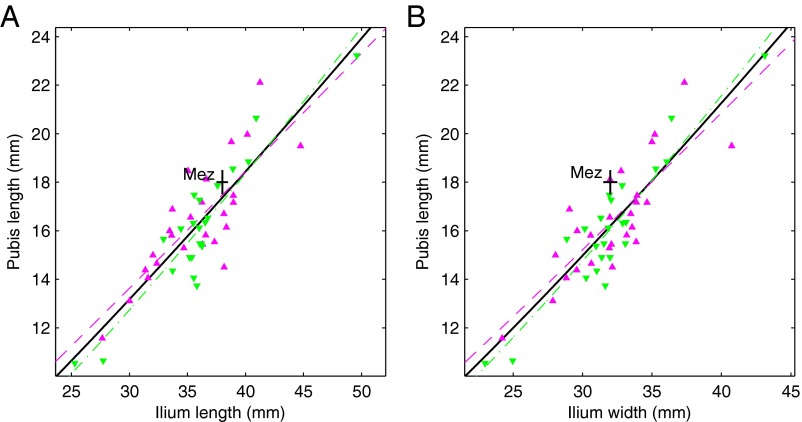
In our fetal/infant sample, African Americans and European Americans do not show a consistent difference across size (i.e., age) in how long the pubis is relative to the size of the ilium (Fig. 2), which is in line with the similarity between African- and European-American adults in ilium–pubis index (ratio of distance between the anterior- and posterior-superior iliac spines to pubis length) (32). Adult Neandertals have a long pubis (33–35), and this characteristic is already present in Mezmaiskaya 1 (Fig. 2).
Fig. 2.
Relative pubis length. (A) Pubis length vs. ilium length and (B) pubis length vs. ilium width (Table S1 includes measurement descriptions). The purple triangles are recent African Americans, the green inverted triangles are recent European Americans, and the black plus sign is the Mezmaiskaya 1 (Mez) Neandertal. The plus-sign horizontal and vertical dimensions are ±0.5 mm. The purple dashed curve is the relationship for the African Americans, the green dash-dot curve is the relationship for the European Americans, and the black solid curve is the relationship for all recent humans (Table S6 includes fit statistics).
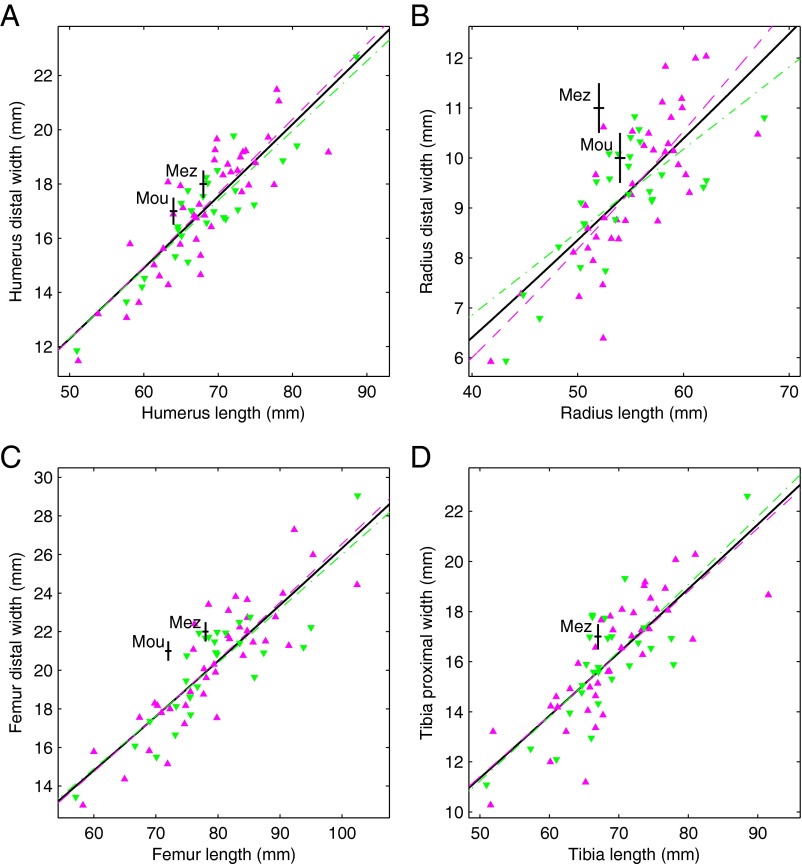
Adult European Americans and African Americans differ, on average, in the shapes of their long bones, with European Americans having thicker shafts and larger articulations relative to shaft length (23, 36). In dry-bone samples of young individuals, it is not possible to compare articulation size to shaft length, because the articulations are mostly or completely cartilaginous. However, it is possible to compare the diaphysis end (i.e., metaphysis) size, which should be related to articulation size, to diaphysis length. Our fetal/infant sample does not show a uniform contrast between European Americans and African Americans in end size to length (Fig. 3). For the humerus (Fig. 3A) and femur (Fig. 3C), African Americans tend to have larger ends relative to length; for the tibia (Fig. 3D), European Americans tend to have larger ends relative to length; and for the radius (Fig. 3B), there is no consistent difference across size (i.e., age) in end size relative to length. Adult Neandertals have thick shafts and large articulations relative to shaft length (36–38), and Mezmaiskaya 1 and Le Moustier 2 may anticipate this feature of adult Neandertals by having long bone diaphyses with very large ends relative to length (Fig. 3).
Fig. 3.
Diaphysis end size to length. (A) Humerus, (B) radius, (C) femur, and (D) tibia (Table S1 includes measurement descriptions). The purple triangles are recent African Americans, the green inverted triangles are recent European Americans, and the black plus signs are Neandertals (Mez, Mezmaiskaya 1; Mou, Le Moustier 2). The plus-sign horizontal and vertical dimensions are ±0.5 mm. The purple dashed curve is the relationship for the African Americans, the green dash-dot curve is the relationship for the European Americans, and the black solid curve is the relationship for all recent humans (Table S6 includes fit statistics).
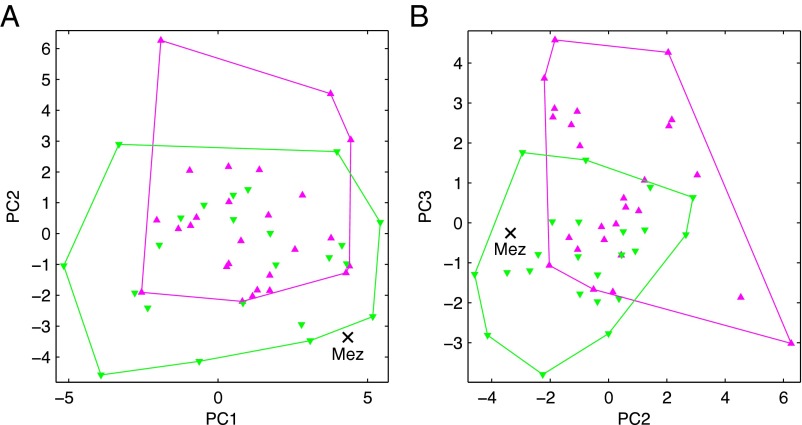
Principal components (PCs) of residuals from the all-recent-human regressions provide a multivariate synthesis of the bivariate analyses (Fig. 4). PC1 encompasses 37% of the variance, and it mainly reflects differences in ilium size relative to femur length and long bone diaphysis end to length dimensions; PC2 encompasses 23% of the variance, and it mainly reflects differences in distal to proximal limb lengths; and PC3 encompasses 19% of the variance, and it mainly reflects differences in pubis length relative to ilium size, ilium length relative to femur length, and radius length relative to humerus length (Table S5). Mezmaiskaya 1 has a highly positive score on PC1 (Fig. 4A), which reflects its large ilium relative to femur length and long bone diaphyses with large ends relative to lengths. Differences between African Americans and European Americans along PC2 and PC3 appear to reflect differences in body proportions, and Mezmaiskaya 1 plots within but near the edge of the European-American convex hull (Fig. 4B).
Fig. 4.
PCs of residuals. (A) PC2 vs. PC1 and (B) PC3 vs. PC2. The purple triangles are recent African Americans, the green inverted triangles are recent European Americans, and the black “X” is the Mezmaiskaya 1 (Mez) Neandertal. The purple and green convex hulls indicate the extent of the African Americans and European Americans, respectively (Table S5 includes the eigenvectors).
Table S5.
Eigenvectors for the PCs
| Relationship for residuals | PC1 | PC2 | PC3 |
| Ilium length vs. femur length | 0.55 | −0.03 | −0.33 |
| Ilium width vs. femur length | 0.40 | 0.02 | −0.24 |
| Radius length vs. humerus length | 0.13 | 0.53 | 0.44 |
| Tibial length vs. femur length | 0.13 | 0.73 | 0.10 |
| Pubis length vs. ilium length | −0.12 | −0.20 | 0.48 |
| Pubis length vs. ilium width | −0.07 | −0.22 | 0.46 |
| Humerus distal width vs. humerus length | 0.29 | −0.09 | 0.26 |
| Radius distal width vs. radius length | 0.21 | −0.13 | 0.10 |
| Femur distal width vs. femur length | 0.48 | −0.10 | 0.27 |
| Tibia proximal width vs. tibia length | 0.36 | −0.25 | 0.20 |
Discussion
We found that many Neandertal postcranial characteristics are present around the time of birth. Neonatal Neandertals already appear to possess the wide body, long pubis, and robust long bones of adult Neandertals. In fact, the only exception we could find was a relatively short radius, which Mezmaiskaya 1 had, whereas Le Moustier 2 had a relatively long radius. This ambiguous result may not be so surprising, given that adult Neandertals show less shortening of the radius relative to the humerus than they do of the tibia relative to the femur (39), and that the Dederiyeh 1 subadult Neandertal has a relatively long radius (18). Unfortunately, the clavicles of Mezmaiskaya 1 and Le Moustier 2 are too fragmentary to reliably estimate the length, but the somewhat older Amud 7 Neandertal seems to already have a clavicle that is long relative to humerus length (14).
Consistent with our results, Golovanova et al. (40) concluded that Mezmaiskaya 1 had relatively short distal limb segments and relatively large ends of the radius and femur in comparison with a recent human infant of similar dental age; and Ponce de León et al. (11) found that Mezmaiskaya 1 had absolutely large distal ends of the humerus and femur, absolutely long ilium and pubis, and a relatively short tibia in comparison with the means of measurements collected by Fazekas and Kósa (41) on a recent Hungarian fetal skeletal sample.
We focused our analyses on a set of linear measurements, but, based on other studies, Neandertals < 1 y of age already seem to show other distinctive postcranial features, including bowed long bones (Kiik Koba 2, Mezmaiskaya 1, Le Moustier 2) (11, 42, 43); robust, rounded rib shafts (Kiik Koba 2) (42); an incipient dorsal axillary sulcus of the scapula (Kiik Koba 2) (44); a medially directed radial tuberosity (Mezmaiskaya 1) (40); subequal proximal and distal thumb diaphyses (Le Moustier 2) (43); an opponens pollicis flange on the first metacarpal (Kiik Koba 2) (37); and very thick long bones (Le Moustier 2) (15). However, other studies appear to indicate that at least some Neandertals developed elevated long-bone cross-sectional properties (14, 45) and a thin superior pubic ramus (16, 46) later in life.
Taken together, current evidence indicates that, with some exceptions [e.g., neurocranial globularity (12, 13), thin superior pubic ramus (16, 18, 46), and perhaps radius/humerus proportions (this study)], skeletal differences between Neandertals and modern humans are largely established by the time of birth. Features that are present on the skeletons of neonatal Neandertals could not have developed in response to mechanical loading associated with adult/older-subadult behaviors (e.g., hunting), so they must either have an evolutionary explanation or result from environmental influences on the fetus (e.g., maternal diet).
If we consider that evolutionary changes are a more likely explanation than fetal environment for skeletal differences between Neandertals and modern humans, climatic adaptation is the best-supported explanation for many aspects of Neandertal postcranial form because Neandertals have body proportions close to present-day humans with ancestry in cold climates (24, 30, 31), it appears that multiple generations are necessary for appreciable changes in body proportions (i.e., an evolutionary timescale is needed) (24, 25), a wide variety of taxa exhibit similar ecological relationships (47–49), and laboratory experiments on human subjects demonstrate that body proportions influence heat loss (50) [see also Churchill (51)]. Recent research, however, has cast some doubt on this explanation. Because the modern human expansion from Africa that gave rise to recent human groups had a substantial south-to-north component, differences among recent human groups in body proportions could have more to do with population history than climatic adaptation (52–54). Additionally, a wide body may have been the ancestral condition for Homo (7, 55–57), even though the earliest members of our genus presumably lived in warm climates. Consequently, although it remains likely that many postcranial differences between Neandertals and modern humans stem from contrasts in body proportions (21, 36, 38, 58), further research will be necessary to establish whether climate played an important role in shaping these differences in body proportions.
Finally, it is important to note that, even if climatic adaptation explains Neandertal body proportions, climatic adaptation alone cannot explain a long pubis relative to dimensions of the pelvic inlet (59, 60). The alternative explanations for a long pubis include a posteriorly positioned acetabulum related to differences in gait between Neandertals and modern humans (60), or a wide pelvis coupled with a transversely oval outlet of the birth canal (as opposed to the anteroposteriorly oval outlets typical of modern humans), because Neandertals had a different birth process than modern humans (59).
Materials and Methods
Mezmaiskaya 1 and Le Moustier 2 Neandertals.
Mezmaiskaya Cave is located in the northwestern Caucasus ∼50 km south of the city of Maikop (Russia). Mezmaiskaya 1 consists of 141 identifiable postcranial bones, a cranium and mandible, and 14 dental crowns of deciduous teeth. The skeleton was recovered in anatomical association from the lowermost 3–5 cm of level 3, the oldest Middle Paleolithic layer (Figs. S1 and S2). Detailed stratigraphic assessments clearly indicate that the Mezmaiskaya 1 skeleton and level 3 were deposited at the same time, both dating to very close to or greater than the effective measurement limit of radiocarbon (∼50 ka) and likely to 70–60 ka based on electron spin resonance mean early and late uptake model determinations for level 3 (11, 40, 61, 62).
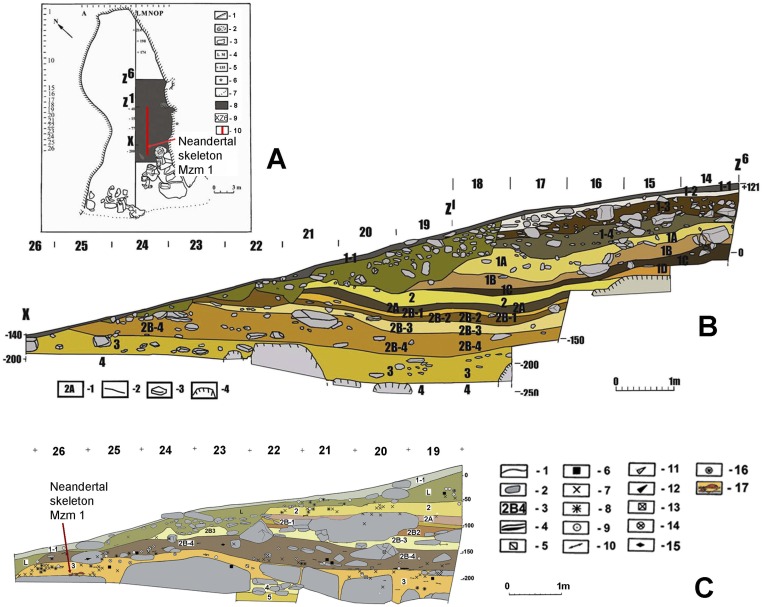
Fig. S1.
Mezmaiskaya cave. Stratigraphy of the cave and the stratigraphic position of the Mezmaiskaya 1 skeleton. (A) Plan of the Mezmaiskaya cave indicating bedrock (1), flowstones (2) and limestone blocks (3), grid position (4), measurements of the modern surface along the cave axis (5), datum (6), dripline (7), 1987–2006 excavation area (8), and indicators for the main longitudinal profile along the cave axis (9) shown in Fig. S1B. The vertical red line (10) indicates the position of the microprofile shown in Fig. S1C. (B) Section XZ6 shows the succession of levels: 1–1 and 1–2 (Holocene), 1–3 through 1C (Epi-Paleolithic and Upper Paleolithic), 1D (volcanic ash), 2–3 (Middle Paleolithic), and 4 (sterile). Legend: 1 and 2, level designations and limits of levels; 3 and 4, stones and blocks. (C) Microprofile constructed along the quadrant line M/east, from M26 through M19, which covers the area where the Mezmaiskaya 1 skeleton was found. The profile shows stones, limestone blocks, and stone artifacts found in situ (bones are not shown in this profile because of the very dense concentration of the remains) from the eastern semiquadrant of line M; it documents the exact stratigraphic position of the Mezmaiskaya 1 skeleton relative to the levels excavated in the area. Levels: 1–1 (Holocene); L, erosional stratum; 2–3 (Middle Paleolithic); and 4–5 (sterile strata). Legend: 1, limits of levels; 2, stones; 3, level designations; 4, charcoal lenses; 5–16, stone artifacts of various types; and 17, Mezmaiskaya 1 skeleton. Image courtesy of L.V.G.
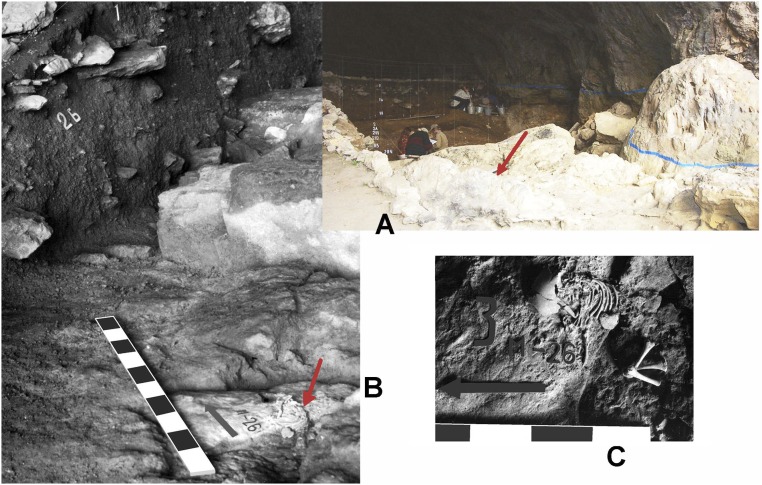
Fig. S2.
Mezmaiskaya 1 Neandertal skeleton. (A) View from the southwest of the 1987–2001 excavation area; the red arrow points to where the Mezmaiskaya 1 skeleton was found in 1993. (B) View from the south-southwest of the Mezmaiskaya 1 skeleton, which was found in quadrant M26 at the bottom of level 3; the red arrow points to the skeleton. (C) View from the west of the Mezmaiskaya 1 skeleton, which is lying in anatomical arrangement; the black arrow points to the north. A and B images courtesy of L.V.G.; image C courtesy of ref. 40, © 1999 by Current Anthropology.
The rock shelters of Le Moustier are located in the Dordogne ∼45 km southeast of Périgueux (France). The Le Moustier 2 skeleton comes from layer J through the top of layer H of the lower rock shelter, which contained Typical Mousterian (layer J) and Discoid Mousterian (top of layer H) artifacts (Figs. S3 and S4). Based on thermoluminescence dating of level J, Le Moustier 2 dates to ∼40 ka BP (43, 63–66).
Fig. S3.
Le Moustier lower rock shelter. View of the upper part of the stratigraphy of the Le Moustier lower rock shelter at the beginning of the 1990s. Following Peyrony’s stratigraphical interpretation (63), Le Moustier 2 was excavated from layer J (“Mousterien typique”) through the top of the layer H (“Mousterien de tradition acheuléenne”) (78–80). Following Gravina and Discamps (66), the upper part of layer H does not contain any Mousterian of Acheulean Tradition; instead, the lithic artifacts should be classified as Discoid Mousterian. Le Moustier 2 is, then, a rare case of a Neandertal primary burial associated with this lithic technocomplex (81). Image courtesy of B.M.
Fig. S4.
Le Moustier 2 Neandertal skeleton.The 2008 restoration of the Le Moustier 2 Neandertal skeleton (Musée national de Préhistoire, Les Eyzies-de-Tayac Sireuil collections). Note that the right humerus and femur are the two long bones still housed at the Musée de l’Homme under the fossil identification “La Ferrassie 4” (43, 77). Picture Philippe Jugie, Musée national de Préhistoire, Les Eyzies-de-Tayac Sireuil. © MNP (Musée national de Préhistoire-Les Eyzies), Dist.RMN, photograph Philippe Jugie.
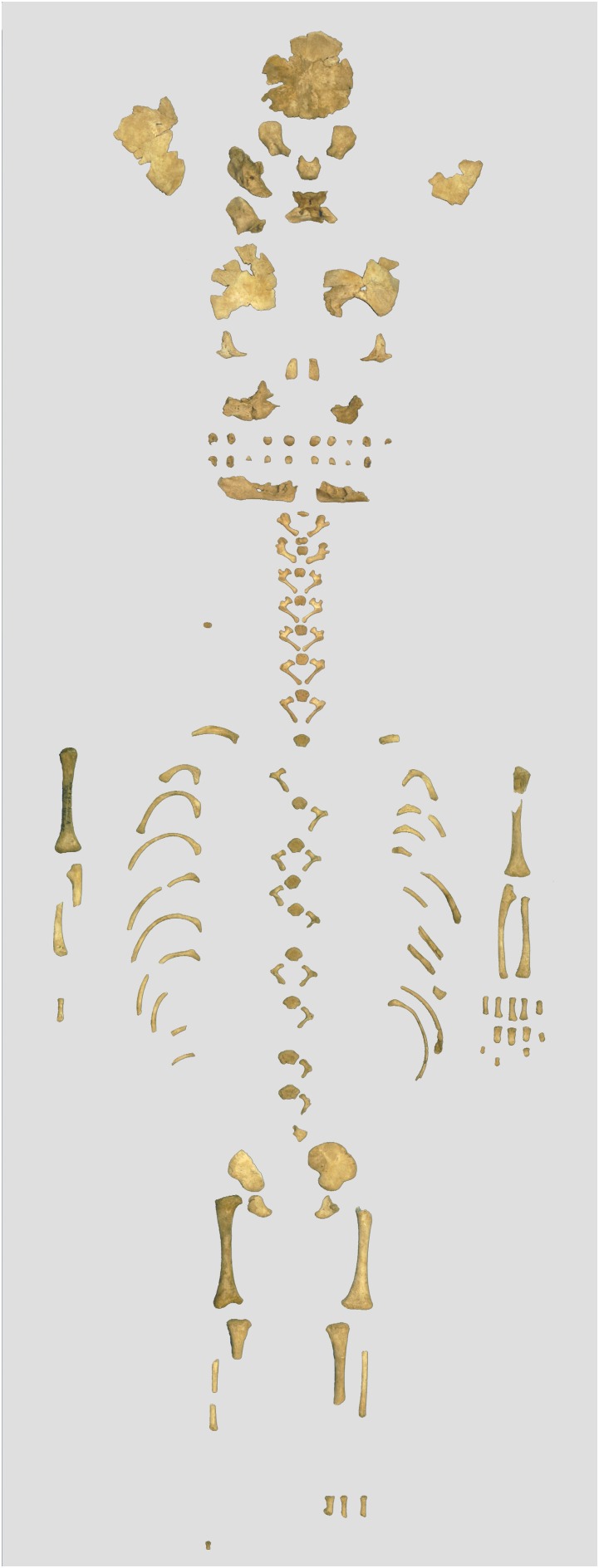
These two fairly complete skeletons are the best preserved Neandertal neonates, and among the most complete Neandertals of any age (Figs. S2 and S4). We were able to collect the full measurement set on Mezmaiskaya 1 and all of the measurements except pubis length and tibia length on Le Moustier 2 (Table S2). The SI Text includes more details about the context and preservation of the Mezmaiskaya 1 and Le Moustier 2 Neandertals.
Recent Human Sample.
The recent human sample is from the fetal skeletal collection housed at the National Museum of Natural History (Smithsonian). Various medical practitioners collected the fetuses, mostly from the Baltimore, MD, and Washington, DC, areas, at the end of the 19th and early 20th centuries and donated them to the museum between 1903 and 1917 (67, 68). Based on the documentation associated with the collection, we divided the sample into two groups: African Americans and European Americans, with approximately equal numbers of female and male specimens in each group (Table S3). Age at death is not precisely documented for much of the sample, but, based on femur length (measurement defined in Table S1; minimum, 57 mm; maximum, 102 mm; mean, 79 mm), the individuals ranged from ∼8 mo in utero to ∼4 mo after birth, with the mean approximately birth (41, 69).
Data Collection.
We collected 11 linear measurements of the humerus, radius, ilium, pubis, femur, and tibia (Table S1) on the left and right sides, preservation permitting, on each of the individuals. We analyzed the mean measurement when both sides could be measured. For Mezmaiskaya 1 and Le Moustier 2 (Table S2), we rounded each measurement to the nearest millimeter and indicated ±0.5 mm on all of the graphs so as not to convey an undue sense of precision when visually comparing these specimens to recent humans.
T.D.W. collected the measurements of the recent humans directly with calipers. Mezmaiskaya 1 was scanned with a Skyscan 1172 micro-CT system (resolution ∼35µm for all dimensions), and Le Moustier 2 was scanned with a BIR ACTIS 225/300 industrial CT scanner (resolution of 20–30 µm for all dimensions). H.C., J.-J.H, B.M., and T.D.W. collected the measurements of Mezmaiskaya 1 and Le Moustier 2 directly with calipers and from CT scans with the Avizo (FEI) and Tivmi software packages.
Statistical Analyses.
We fit nonlinear regressions of the form y = axb (power laws) to the data in the bivariate graphs, where a and b are constants, and y and x are the response and predictor variables, respectively. For each graph, we fit three separate curves: for the African Americans, European Americans, and all of the recent humans (i.e., all individuals except Mezmaiskaya 1 and Le Moustier 2). As with ratios, these curves allow relative dimensions to be compared, but they have the advantage of allowing the ratio to vary with size (i.e., age). (An exponent [b] equal to one indicates that the ratio between the two variables remains constant.) Because relative dimensions are being compared, our analyses are independent of age estimates, which is advantageous given evidence that Neandertals may have matured faster than modern humans (70). Individuals with missing data for one (or both) of the variables were excluded from the particular bivariate analysis. Table S6 provides fit statistics for the bivariate analyses.
Table S6.
Fit statistics for the bivariate plots
| Relationship | African Americans | European Americans | All recent humans | |||||||||
| n | a | b | r2 | n | a | b | r2 | n | a | b | r2 | |
| Ilium length vs. femur length | 38 | 0.49 | 0.97 | 0.83 | 27 | 0.41 | 1.02 | 0.80 | 65 | 0.46 | 0.99 | 0.80 |
| Ilium width vs. femur length | 38 | 0.46 | 0.96 | 0.86 | 27 | 0.42 | 0.99 | 0.85 | 65 | 0.44 | 0.97 | 0.85 |
| Radius length vs. humerus length | 39 | 1.61 | 0.84 | 0.90 | 28 | 1.47 | 0.85 | 0.89 | 67 | 1.55 | 0.84 | 0.88 |
| Tibia length vs. femur length | 38 | 1.08 | 0.95 | 0.95 | 27 | 1.44 | 0.88 | 0.94 | 65 | 1.21 | 0.92 | 0.94 |
| Pubis length vs. ilium length | 26 | 0.38 | 1.05 | 0.64 | 23 | 0.17 | 1.27 | 0.86 | 49 | 0.25 | 1.17 | 0.75 |
| Pubis length vs. ilium width | 26 | 0.36 | 1.10 | 0.66 | 23 | 0.17 | 1.32 | 0.86 | 49 | 0.24 | 1.22 | 0.77 |
| Humerus proximal width vs. humerus length | 40 | 0.18 | 1.08 | 0.72 | 28 | 0.22 | 1.03 | 0.82 | 68 | 0.20 | 1.06 | 0.75 |
| Radius proximal width vs. radius length | 39 | 0.04 | 1.39 | 0.61 | 28 | 0.19 | 0.97 | 0.54 | 67 | 0.08 | 1.19 | 0.58 |
| Femur proximal width vs. femur length | 39 | 0.13 | 1.15 | 0.75 | 28 | 0.17 | 1.09 | 0.73 | 67 | 0.15 | 1.13 | 0.74 |
| Tibia distal width vs. tibia length | 39 | 0.18 | 1.06 | 0.65 | 27 | 0.14 | 1.13 | 0.63 | 66 | 0.16 | 1.08 | 0.64 |
Statistics refer to the nonlinear regression of the form y = axb, where y and x are the response and predictor variables respectively; n is the sample size and r2 is the coefficient of determination.
To provide a multivariate synthesis of the bivariate analyses, we performed PC analysis (PCA) of the residuals of the individuals from the all-recent-human regressions (i.e., the residuals from a particular bivariate analysis correspond to a variable in the PCA). We performed the PCA on the covariance matrix, and individuals with any missing data were excluded from this analysis. We performed all statistical analyses in Matlab (Mathworks).
SI Text
Mezmaiskaya 1.
Mezmaiskaya cave is located 1,310 m above sea level along a small tributary of the Kurdjips River, ∼50 km south of the city of Maikop (northwestern Caucasus, Russia). L.V.G. has excavated the site since 1987. Currently, 6 Holocene and 20 Pleistocene strata are identified over an ∼80-m2 excavation area (Fig. S1 A and B), including 7 Middle Paleolithic levels dating to ca. 70–40 ka BP (40, 61, 62, 71, 72), 6 Upper Paleolithic and 2 Epi-Paleolithic levels dating to ca. 39–12 ka calibrated BP (73–75), and 6 post-Paleolithic levels, including a Neolithic level deeper in the cave that was found in 2011. The Neandertal fossils recovered from Mezmaiskaya cave are: the skeleton of a Neandertal neonate (Mezmaiskaya 1), which was discovered in 1993 in level 3, the oldest Middle Paleolithic deposits; an isolated permanent tooth (Mezmaiskaya 3), which was found later and is also from level 3; and skull fragments of a Neandertal child (Mezmaiskaya 2), which were found in 1994 in level 2, the uppermost Middle Paleolithic deposits.
The Mezmaiskaya 1 almost-complete skeleton was recovered in situ and in anatomical arrangement from the lowermost 3–5 cm of level 3 in the cave entrance area where most Pleistocene layers were destroyed by erosion (Figs. S1 A and C and S2). The skeleton occupied an area of 20 × 30 cm in quadrant M26, atop a large limestone block, and it was overlain by the Middle Paleolithic levels 3 and 2B–4 and a modern soil (level 1–1). Significantly, level 2B–4 was preserved close to the eastern wall of the cave, including quadrant M26 (Fig. S1C), but it gradually thinned toward the cave axis and is not present on section XZ6 (Fig. S1B). In the area where the skeleton was found, the sediments of levels 3 and 2B–4 underwent a strong calcium-carbonate cementation to the state of a rugged breccia in the lowermost part of level 3. Thanks to strong calcite cementation, which encased large portions of the Mezmaiskaya 1 remains, most of the bones were found in an excellent state of preservation and suffered only minor postdepositional disturbance. The skull was partly damaged and collapsed, but all of the cranial elements were preserved in close anatomical association (Fig. S2C). The left scapula, humerus, radius, most of the vertebral column and ribs, and the pelvis were preserved in anatomical arrangement. The long bones of the legs were disarticulated, and the metatarsals were gnawed by rodents. The body was deposited on the right side in what appears to have been a flexed position, head oriented to the north (inside the cave), with the left arm extended along the trunk and flexed slightly at the elbow. The position of the legs was difficult to determine, but the anatomical association between the spine and pelvis suggests that the legs were bent at the knees. Analyses of linear craniodental and postcranial dimensions of the Mezmaiskaya 1 specimen in comparison with present-day human neonates suggest that the Mezmaiskaya individual died approximately 2 wk after birth (11, 13).
No traces of a burial pit were observed, and no artifacts or faunal remains were found in the lowermost part of level 3 in the immediate vicinity of the skeleton. However, small (2–3 mm) charcoal fragments were recovered among the skeleton but were lacking in adjacent parts of level 3. The skeleton’s good preservation with no evidence of scavenger marks on the bones suggest an intentional burial. Several microprofiles constructed across the skeleton area (figures S1C and S2 in ref. 11) indicate that the burial was made in the initial period of Neandertal occupation of the cave (level 3), before a significant accumulation of artifacts and faunal remains in the sediments of this level. Taken together, the evidence suggests that the Mezmaiskaya 1 Neandertal neonate was buried in a flexed position in a shallow pit or directly on the surface of a limestone block and covered with the surrounding sediments, and postdepositional processes completely obliterated traces of the burial. Mezmaiskaya 1 is one of the most complete and best-preserved Neandertal skeletons of any age and the youngest (2 wk after birth) Neandertal individual found in the world.
Le Moustier 2.
Le Moustier is a small village situated 10 km upriver from Les Eyzies-de-Tayac Sireuil at the crossing of the Vèzère and Vimont valleys in the Dordogne (southwest region of France). There, three different archaeological sites are located on a Coniacian limestone cliff. Two of them are very well known: the Upper (or Classical) and the Lower (or Peyrony) rock shelters (Fig. S3). In May 1914, during his excavations of the Lower shelter, D. Peyrony found a burial of a very young child (Le Moustier 2) within a Mousterian layer (layer H). Very quickly, he sent (at least) two long bones to M. Boule to obtain an age at death estimate for the individual. He received Boule’s answer a few days later: it was a neonate. Following Peyrony’s stratigraphical interpretation, the burial of Le Moustier 2 was excavated from layer J through to the top of the layer H. All of the information recorded by Peyrony during the excavation suggests an intentional Mousterian primary interment (43, 63, 76). Unfortunately, from Peyrony’s records, we do not have any precise data about the skeleton’s position within the burial pit. Then, for a variety of reasons, although Peyrony mentioned Le Moustier 2 in different publications, the fossil was forgotten by the scientific community, or considered to be lost during the first World War (77). In September 1996, one of the present authors (B.M.), while conducting an inventory of the human remains preserved in the collections of the Musée national de Préhistoire at les Eyzies-de-Tayac Sireuil, found small sediment blocks and isolated neonatal human remains in a wooden drawer among the collections belonging to Peyrony’s excavations of the Le Moustier Lower shelter. With the authorization of the French Ministry of Culture, over a period of 150 d (between 1997 and 2001), the sediment blocks containing most of the neonate’s bones were excavated and restored. In the end, it was possible to present to the scientific community one of the best-preserved Neandertal skeletons, including adults (Fig. S4) (65). Today, the Le Moustier 2 original fossil, a Neandertal neonate, which, based on present-day human standards, cannot be older than 4 mo after birth, is one of the most beautiful hominin fossils presented at the permanent exhibition of the Musée national de Préhistoire.
Acknowledgments
We thank T. Steele, Y. Rak, and two reviewers for helpful comments and D. Hunt for access to and assistance with collections. This study was funded by the Max Planck Society, the Aquitaine Regional Council (Transitions Program, convention 20051403003AB), and the National Research Agency’s Future Investments program (ANR-10-LABX-52 - Bordeaux Archaeological Sciences LabEx cluster of excellence, NEMO project).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523677113/-/DCSupplemental.
References
- 1.Pearson OM. Postcranial remains and the origin of modern humans. Evol Anthropol. 2000;9:229–247. [Google Scholar]
- 2.Spoor F, Hublin J-J, Braun M, Zonneveld F. The bony labyrinth of Neanderthals. J Hum Evol. 2003;44(2):141–165. doi: 10.1016/s0047-2484(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 3.Harvati K, Frost SR, McNulty KP. Neanderthal taxonomy reconsidered: Implications of 3D primate models of intra- and interspecific differences. Proc Natl Acad Sci USA. 2004;101(5):1147–1152. doi: 10.1073/pnas.0308085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinkaus E. Modern human versus Neandertal evolutionary distinctiveness. Curr Anthropol. 2006;47:597–620. [Google Scholar]
- 5.Arsuaga JL, et al. Neandertal roots: Cranial and chronological evidence from Sima de los Huesos. Science. 2014;344(6190):1358–1363. doi: 10.1126/science.1253958. [DOI] [PubMed] [Google Scholar]
- 6.Hublin J-J. Anthropology. How to build a Neandertal. Science. 2014;344(6190):1338–1339. doi: 10.1126/science.1255554. [DOI] [PubMed] [Google Scholar]
- 7.Arsuaga JL, et al. Postcranial morphology of the middle Pleistocene humans from Sima de los Huesos, Spain. Proc Natl Acad Sci USA. 2015;112(37):11524–11529. doi: 10.1073/pnas.1514828112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponce de León MS, Zollikofer CPE. Neanderthal cranial ontogeny and its implications for late hominid diversity. Nature. 2001;412(6846):534–538. doi: 10.1038/35087573. [DOI] [PubMed] [Google Scholar]
- 9.Krovitz GE. Shape and growth differences between Neandertals and modern humans: Grounds for a species-level distinction? In: Thompson JL, Krovitz GE, Nelson AJ, editors. Patterns of Growth and Development in the Genus Homo. Cambridge Univ Press; Cambridge, UK: 2003. pp. 320–342. [Google Scholar]
- 10.Bastir M, O’Higgins P, Rosas A. Facial ontogeny in Neanderthals and modern humans. Proc Biol Sci. 2007;274(1614):1125–1132. doi: 10.1098/rspb.2006.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponce de León MS, et al. Neanderthal brain size at birth provides insights into the evolution of human life history. Proc Natl Acad Sci USA. 2008;105(37):13764–13768. doi: 10.1073/pnas.0803917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunz P, Neubauer S, Maureille B, Hublin J-J. Brain development after birth differs between Neanderthals and modern humans. Curr Biol. 2010;20(21):R921–R922. doi: 10.1016/j.cub.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Gunz P, et al. A uniquely modern human pattern of endocranial development. Insights from a new cranial reconstruction of the Neandertal newborn from Mezmaiskaya. J Hum Evol. 2012;62(2):300–313. doi: 10.1016/j.jhevol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Odwak H. Long bone robusticity and claviculo-humeral proportions of the Amud 7 Neandertal baby. Am J Phys Anthropol. 2000;(Supplement 30):241. [Google Scholar]
- 15.Maureille B. The rediscovery of Le Moustier 2 Neandertal specimen. In: Ullrich H, editor. The Neanderthal Adolescent Le Moustier 1: New Aspects, New Results. Staatliche Museenzu Berlin–Preussischer Kulturbesitz; Berlin: 2005. pp. 63–72. [Google Scholar]
- 16.Akazawa T, et al. Neanderthal infant burial from the Dederiyeh Cave in Syria. Paéorient. 1995;21:77–86. [Google Scholar]
- 17.Akazawa T, Muhesen S, Ishida H, Kondo O, Griggo C. New discovery of a Neanderthal child burial from the Dederiyeh Cave in Syria. Paéorient. 1999;25:129–142. [Google Scholar]
- 18.Kondo O, Dodo Y. The postcranial bones of the Neanderthal child of burial no. 1. In: Akazawa T, Muhesen S, editors. Neanderthal Burials: Excavations of the Dederiyeh Cave, Afrin, Syria. International Research Center for Japanese Studies; Kyoto: 2000. [Google Scholar]
- 19.Arsuaga JL, et al. New Neandertal remains from CovaNegra (Valencia, Spain) J Hum Evol. 2007;52(1):31–58. doi: 10.1016/j.jhevol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Tillier A-M. Facts and ideas in Paleolithic growth studies (paleoauxology) In: Condemi S, Weninger G-C, editors. Continuity and Discontinuity in the Peopling of Europe: One Hundred Fifty Years of Neanderthal Study. Springer; Dordrecht, The Netherlands: 2011. pp. 139–153. [Google Scholar]
- 21.Weaver TD. Out of Africa: Modern human origins special feature: The meaning of Neandertal skeletal morphology. Proc Natl Acad Sci USA. 2009;106(38):16028–16033. doi: 10.1073/pnas.0903864106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruff CB, Walker A. Body size and body shape. In: Walker A, Leakey R, editors. The Nariokotome Homo erectus Skeleton. Harvard Univ Press; Cambridge: 1993. pp. 234–265. [Google Scholar]
- 23.Holliday TW, Falsetti AB. A new method for discriminating African-American from European-American skeletons using postcranial osteometrics reflective of body shape. J Forensic Sci. 1999;44(5):926–930. [PubMed] [Google Scholar]
- 24.Ruff CB. Morphological adaptation to climate in modern and fossil hominids. Yearb Phys Anthropol. 1994;37:65–107. [Google Scholar]
- 25.Holliday TW. Body proportions in Late Pleistocene Europe and modern human origins. J Hum Evol. 1997;32(5):423–448. doi: 10.1006/jhev.1996.0111. [DOI] [PubMed] [Google Scholar]
- 26.Bergmann C. Über die Verhältnisse der Wärmeökonomie der Thiere zu Ihrer Grösse. Göttinger Studien. 1847;3:595–708. [Google Scholar]
- 27.Allen JA. The influence of physical conditions in the genesis of species. Radic Rev. 1877;1:108–140. [Google Scholar]
- 28.Schultz AH. Fetal growth in man and other primates. Q Rev Biol. 1926;1:465–521. [Google Scholar]
- 29.Cowgill LW, Eleazer CD, Auerbach BM, Temple DH, Okazaki K. Developmental variation in ecogeographic body proportions. Am J Phys Anthropol. 2012;148(4):557–570. doi: 10.1002/ajpa.22072. [DOI] [PubMed] [Google Scholar]
- 30.Trinkaus E. Neanderthal limb proportions and cold adaptation. In: Stringer CB, editor. Aspects of Human Evolution, Symposia of the Society for the Study of Human Biology. Vol 21. Taylor and Francis; London: 1981. pp. 187–224. [Google Scholar]
- 31.Holliday TW. Postcranial evidence of cold adaptation in European Neandertals. Am J Phys Anthropol. 1997;104(2):245–258. doi: 10.1002/(SICI)1096-8644(199710)104:2<245::AID-AJPA10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Kimura K. Sex differences of the hip bone among several populations. Okajimas Folia Anat Jpn. 1982;58(4-6):265–276. doi: 10.2535/ofaj1936.58.4-6_265. [DOI] [PubMed] [Google Scholar]
- 33.Stewart TD. Form of the pubic bone in Neanderthal man. Science. 1960;131(3411):1437–1438. doi: 10.1126/science.131.3411.1437. [DOI] [PubMed] [Google Scholar]
- 34.Trinkaus E. Neandertal pubic morphology and gestation length. Curr Anthropol. 1984;25:509–514. [Google Scholar]
- 35.Rosenberg KR. The functional significance of Neandertal pubic length. Curr Anthropol. 1988;29:595–607. [Google Scholar]
- 36.Pearson OM. Activity, climate, and postcranial robusticity: Implications for modern human origins and scenarios of adaptive change. Curr Anthropol. 2000;41(4):569–607. [PubMed] [Google Scholar]
- 37.Trinkaus E. Neandertal postcrania and the adaptive shift to modern humans. In: Trinkaus E, editor. The Mousterian Legacy: Human Biocultural Change in the Upper Pleistocene. Vol 164. British Archaeological Reports International; Oxford: 1983. pp. 165–200. [Google Scholar]
- 38.Weaver TD. The shape of the Neandertal femur is primarily the consequence of a hyperpolar body form. Proc Natl Acad Sci USA. 2003;100(12):6926–6929. doi: 10.1073/pnas.1232340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker MJ, Ortega J, Parmová K, López MV, Trinkaus E. Morphology, body proportions, and postcranial hypertrophy of a female Neandertal from the Sima de las Palomas, southeastern Spain. Proc Natl Acad Sci USA. 2011;108(25):10087–10091. doi: 10.1073/pnas.1107318108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golovanova LV, Hoffecker JF, Kharitonov VM, Romanova G. Mezmaiskaya Cave: A Neanderthal occupation in the northern Caucasus. Curr Anthropol. 1999;40:77–86. [Google Scholar]
- 41.Fazekas IG, Kósa F. Forensic Fetal Osteology. Akadémiai Kiadó; Budapest: 1978. p. 96. [Google Scholar]
- 42.Vlcek E. Postcranial skeleton of a Neandertal child from Kiik-Koba, U.S.S.R. J Hum Evol. 1973;2:537–544. [Google Scholar]
- 43.Maureille B. La redécouverte du nouveau-né néandertalien Le Moustier 2. PALEO. 2002;14:221–238. [Google Scholar]
- 44.Trinkaus E. Kiik-Koba 2 and Neandertal axillary border ontogeny. Anthropol Sci. 2008;116:231–236. [Google Scholar]
- 45.Cowgill LW. The ontogeny of Holocene and Late Pleistocene human postcranial strength. Am J Phys Anthropol. 2010;141(1):16–37. doi: 10.1002/ajpa.21107. [DOI] [PubMed] [Google Scholar]
- 46.Tompkins RL, Trinkaus E. La Ferrassie 6 and the development of Neandertal pubic morphology. Am J Phys Anthropol. 1987;73(2):233–239. doi: 10.1002/ajpa.1330730210. [DOI] [PubMed] [Google Scholar]
- 47.Ashton KG, Tracy MC, de Queiroz A. Is Bergmann’s rule valid for mammals? Am Nat. 2000;156:390–415. doi: 10.1086/303400. [DOI] [PubMed] [Google Scholar]
- 48.Freckleton RP, Harvey PH, Pagel M. Bergmann’s rule and body size in mammals. Am Nat. 2003;161(5):821–825. doi: 10.1086/374346. [DOI] [PubMed] [Google Scholar]
- 49.Harcourt AH. Human Biogeography. Univ California Press; Berkeley: 2012. p. 328. [Google Scholar]
- 50.Tilkens MJ, Wall-Scheffler C, Weaver TD, Steudel-Numbers K. The effects of body proportions on thermoregulation: An experimental assessment of Allen’s rule. J Hum Evol. 2007;53(3):286–291. doi: 10.1016/j.jhevol.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Churchill SE. Thin on the Ground: Neandertal Biology, Archeology, and Ecology. Wiley-Blackwell; Ames, IA: 2014. [Google Scholar]
- 52.Betti L, von Cramon-Taubadel N, Manica A, Lycett SJ. Global geometric morphometric analyses of the human pelvis reveal substantial neutral population history effects, even across sexes. PLoS One. 2013;8(2):e55909. doi: 10.1371/journal.pone.0055909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Betti L, von Cramon-Taubadel N, Manica A, Lycett SJ. The interaction of neutral evolutionary processes with climatically-driven adaptive changes in the 3D shape of the human oscoxae. J Hum Evol. 2014;73:64–74. doi: 10.1016/j.jhevol.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Roseman CC, Auerbach BM. Ecogeography, genetics, and the evolution of human body form. J Hum Evol. 2015;78:80–90. doi: 10.1016/j.jhevol.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Arsuaga J-L, et al. A complete human pelvis from the Middle Pleistocene of Spain. Nature. 1999;399(6733):255–258. doi: 10.1038/20430. [DOI] [PubMed] [Google Scholar]
- 56.Simpson SW, et al. A female Homo erectus pelvis from Gona, Ethiopia. Science. 2008;322(5904):1089–1092. doi: 10.1126/science.1163592. [DOI] [PubMed] [Google Scholar]
- 57.Bonmatí A, et al. Middle Pleistocene lower back and pelvis from an aged human individual from the Sima de los Huesos site, Spain. Proc Natl Acad Sci USA. 2010;107(43):18386–18391. doi: 10.1073/pnas.1012131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trinkaus E, Ruff CB, Churchill SE. Upper limb versus lower limb loading patterns among Near Eastern Middle Paleolithic hominids. In: Akazawa T, Aoki K, Bar-Yosef O, editors. Neanderthals and Modern Humans in Western Asia. Plenum; New York: 1998. pp. 391–404. [Google Scholar]
- 59.Weaver TD, Hublin J-J. Neandertal birth canal shape and the evolution of human childbirth. Proc Natl Acad Sci USA. 2009;106(20):8151–8156. doi: 10.1073/pnas.0812554106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rak Y. The pelvis. In: Bar-Yosef O, Vandermeersch B, editors. Le Squelette Moustérien de Kébara. CNRS Editions; Paris: 1991. pp. 147–166. [Google Scholar]
- 61.Skinner AR, et al. ESR dating at Mezmaiskaya Cave, Russia. Appl Radiat Isot. 2005;62(2):219–224. doi: 10.1016/j.apradiso.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Pinhasi R, Higham TFG, Golovanova LV, Doronichev VB. Revised age of late Neanderthal occupation and the end of the Middle Paleolithic in the northern Caucasus. Proc Natl Acad Sci USA. 2011;108(21):8611–8616. doi: 10.1073/pnas.1018938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peyrony D. Le Moustier: Sesgisements, ses industries, ses couches géologiques. Rev Anthropol. 1930;40:3–76,155–176. [Google Scholar]
- 64.Valladas H, Geneste JM, Joron JL, Chadelle JP. Thermoluminescence dating of Le Moustier (Dordogne, France) Nature. 1986;322:452–454. [Google Scholar]
- 65.Maureille B. A lost Neanderthal neonate found. Nature. 2002;419(6902):33–34. doi: 10.1038/419033a. [DOI] [PubMed] [Google Scholar]
- 66.Gravina B, Discamps E. MTA-B or not to be? Recycled bifaces and shifting hunting strategies at Le Moustier and their implication for the late Middle Palaeolithic in southwestern France. J Hum Evol. 2015;84:83–98. doi: 10.1016/j.jhevol.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Gindhart PS. An early twentieth-century skeleton collection. J Forensic Sci. 1989;34(4):887–893. [PubMed] [Google Scholar]
- 68.Huxley AK. Gestational age discrepancies due to acquisition artifact in the forensic fetal osteology collection at the National Museum of Natural History, Smithsonian Institution, USA. Am J Forensic Med Pathol. 2005;26(3):216–220. doi: 10.1097/01.paf.0000176281.96564.29. [DOI] [PubMed] [Google Scholar]
- 69.Scheuer L, Black S. Developmental Juvenile Osteology. Academic; San Diego: 2000. [Google Scholar]
- 70.Smith TM, et al. Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proc Natl Acad Sci USA. 2010;107(49):20923–20928. doi: 10.1073/pnas.1010906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Golovanova L, et al. Un site Micoquien est-Europeem du Caucase du nord (resultants préliminaires de l’étude de la grotte Mezmaiskaya, les fouilles des années 1987-1993) Anthropologie (Paris) 1998;102:45–66. [Google Scholar]
- 72.Golovanova L. Les hommes de Néandertal du Caucase du Nord: Entre l’ouest et l’est. Anthropologie (Paris) 2015;119:45–66. [Google Scholar]
- 73.Golovanova L, et al. The early Upper Paleolithic in Northern Caucasus (new data from Mezmaiskaya cave, excavation 1997) Eurasian Prehistory. 2006;4:43–78. [Google Scholar]
- 74.Golovanova L, Doronichev V. The Early Upper Paleolithic of the Caucasus in the West Eurasian Context. In: Otte M, Shidrang S, Flas D, editors. L’Aurignacien de la Grotte Yaftehet son Contexte (Fouilles 2005-2008) Editions ERAUL 132, Univ de Liège, Liège; Belgium: 2012. pp. 137–160. [Google Scholar]
- 75.Golovanova L, et al. The epipaleolithic of the Caucasus after the last glacial maximum. Quat Int. 2014;337:189–224. [Google Scholar]
- 76.Maureille B, Turq A. The Neanderthal adolescent Le Moustier 1: New Aspects, New Results. Staatliche Museenzu Berlin–Preussischer Kulturbesitz; Berlin: 2005. Le Moustier sites’ excavations and their importance in French archaeology; pp. 21–28. [Google Scholar]
- 77.Heim J-L. Les Enfants Néandertaliens de La Ferrassie: Etude Anthropologique et Analyse Ontogenique des Hommes de Neandertal. Masson; Paris: 1982. [Google Scholar]
- 78.Bordes F, Bourgon M. Le complexe Moustérien: Moustérien, levalloisien et tayacien. Anthropologie (Paris) 1951;55:1–23. [Google Scholar]
- 79.Laville H, Rigaud J-P. L’abriinférieur du Moustier (Dordogne): Précisions stratigraphiques et chronologiques. Comptes Rendus Acad Sci. 1973;276:3097–3100. [Google Scholar]
- 80.Laville H, Rigaud J-P, Sackett J. Rock Shelters of the Perigord: Geological Stratigraphy and Archaeological Succession. Academic; New York: 1980. [Google Scholar]
- 81.Maureille B, Tillier A-M. Répartition géographique et chronologique des sepultures néandertaliennes. In: Vandermeersch B, Cleyet-Merle J-J, Jaubert J, Maureille B, Turq A, editors. Première Humanité, Gestes Funéraires des Néandertaliens. Réunion des Musées Nationaux; Paris: 2008. pp. 66–74. [Google Scholar]