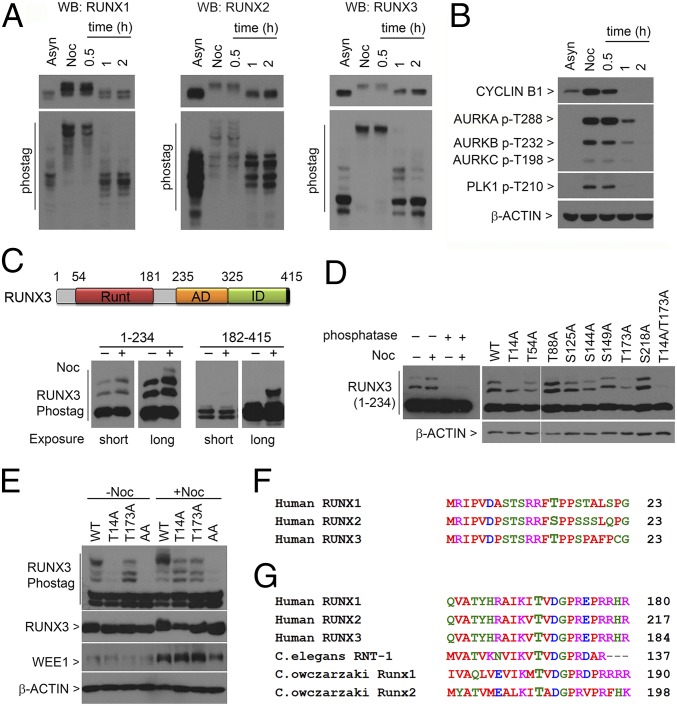
Fig. 1.
T14 and T173 are important for RUNX3 hyperphosphorylation in mitosis. (A) U2OS cells were synchronized with nocodazole (Noc), collected by mitotic shake-off, replated in media without nocodazole, and harvested at the indicated times. Lysates were electrophoresed in conventional SDS/PAGE (Top) and Phos-tag gels (Bottom) for immunoblotting with the indicated antibodies. Asyn, asynchronous. (B) Lysates from A were immunoblotted with the indicated antibodies. (C, Top) Schematic of human RUNX3. Runt domain, DNA-binding domain; AD, activation domain; ID, inhibitory domain. (Bottom) Flag-tagged truncation constructs of RUNX3 spanning amino acids 1–234 and 182–415 were transfected into COS7 cells. After 28 h, cells were cultured with (+) or without (−) nocodazole. Nocodazole-treated cells were harvested by mitotic shake-off. Lysates were immunoblotted with anti-Flag antibodies. Short and long exposure times were indicated. (D) Phos-tag electrophoresis of lysates containing Flag-RUNX3 (amino acids 1–234). (Left) Lysates treated with lambda-protein phosphatase. (Right) Point mutants of Flag-RUNX3 (amino acids 1–234) were transfected into COS7 cells and treated with nocodazole. Immunoblots were probed with anti-Flag antibodies. WT, wild type. (E) T14 and T173 mutations were introduced into full-length Flag-tagged RUNX3 and transfected into COS7 cells. Transfectants were treated as in C and immunoblotted with the indicated antibodies. (F and G) Alignments of the regions flanking T14 and T173 in human RUNX3 with human RUNX1, RUNX2, C. elegans RNT-1, and C. owczarzaki RUNX1 and RUNX2. Protein sequence alignments were done with ClustalW (www.ebi.ac.uk/Tools/msa/clustalw2).