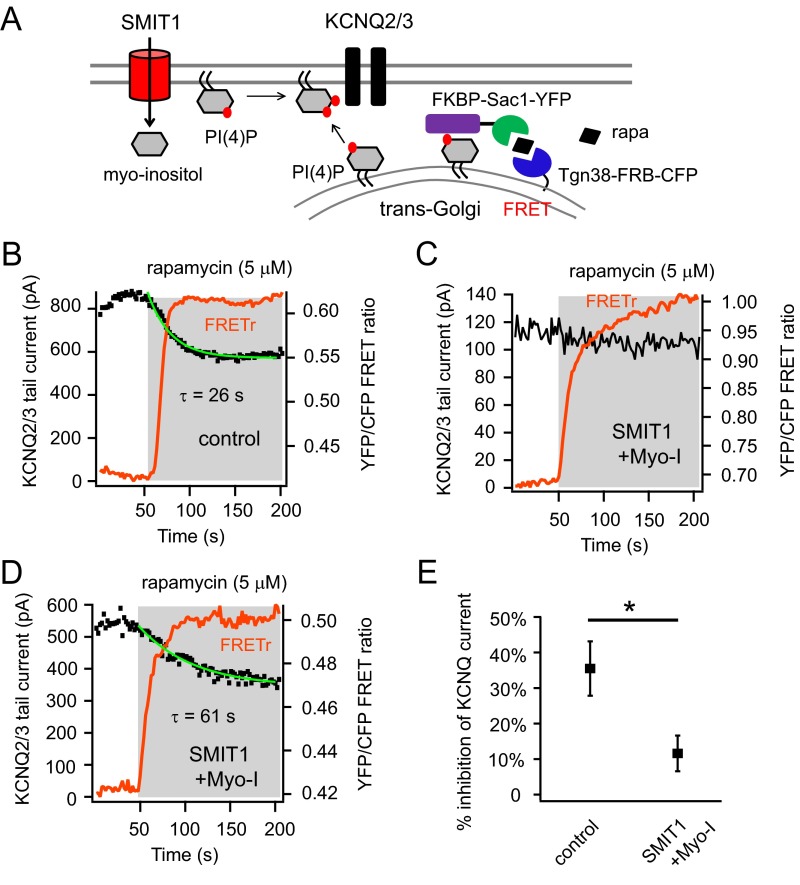
Fig. S5.
PI(4)P metabolism was altered by SMIT1 and myo-inositol. Related to Fig. 4. (A) Cartoon illustrating the hydrolysis of Golgi-localized PI(4)P using a rapamycin-recruitable lipid 4 phosphatase Sac1 targeted to the trans-Golgi network. In contrast to the translocation of Sac1 to the plasma membrane using Lyn11-FRB, we recruited Sac1 to the trans-Golgi network using an anchored Tgn38-FRB while maintaining the other features of the simultaneous photometry and electrophysiology recordings. Applying rapamycin in this situation resulted in specific hydrolysis of PI(4)P at the trans-Golgi network. (B and C) Representative experiments of simultaneous patch-clamp recordings and photometric FRET ratio measurements, showing the reduction of KCNQ2/3 current for the control and very little inhibition for the majority of cells treated with SMIT1 and myo-inositol after applying 5 μM rapamycin. We found the reduction of KCNQ current after rapamycin application was significantly blunted by SMIT1 and myo-inositol. (D) Some heterogeneity for these results was observed suggesting that the contribution of the PI(4)P pool to plasma membrane PI(4,5)P2 varies among individual cells. We compared the slopes of the KCNQ2/3 current inhibition of a control cell and a SMIT1 cell supplemented with myo-inositol that exhibited similar percentages of inhibition; under these conditions the time constant for current inhibition was much slower with SMIT1 and myo-inositol. D shows the representative recordings of KCNQ2/3 current for cells treated with SMIT1 and myo-inositol with the comparable percentage of current inhibition but a much slower kinetics compared with the control in B. (E) Summary of the percentages of inhibition of KCNQ2/3 current when Golgi PI(4)P is hydrolyzed (n = 5). Data are means ± SEM.