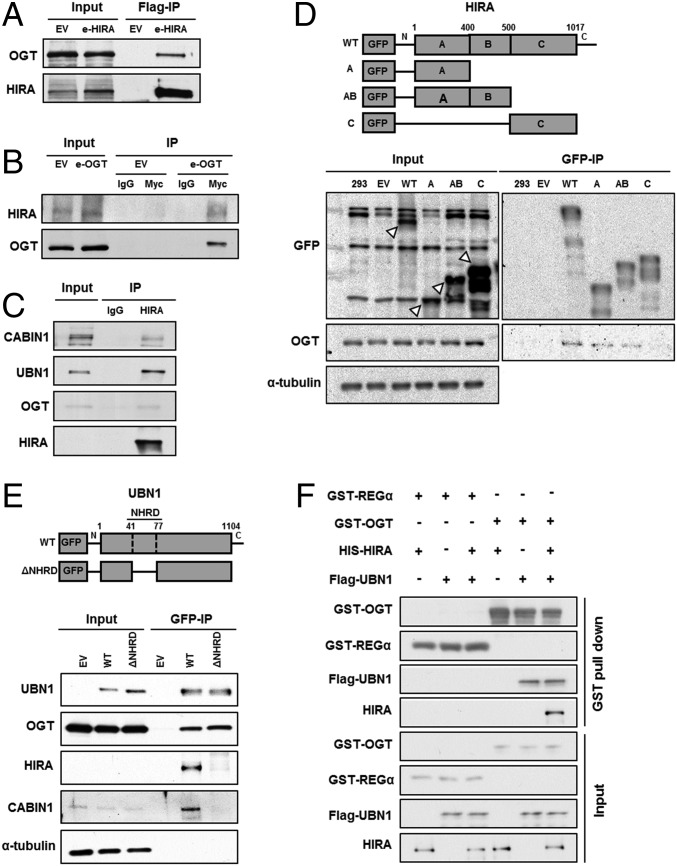
Fig. 1.
OGT interacts with the HIRA complex. (A) Exogenously expressed HIRA associates with endogenous OGT. Exogenously expressed Flag-HIRA (e-HIRA) was immunoprecipitated (IP) with Flag M2 beads from 293T cells, and proteins at input and IP samples were analyzed by Western blotting. EV, empty vector. (B) Exogenously expressed OGT (e-OGT) associates with endogenous HIRA. The experiment was performed as described in A except that Myc-OGT was immunoprecipitated with antibodies against the Myc epitope. (C) Endogenous HIRA associates with endogenous OGT. HIRA was immunoprecipitated with antibodies against HIRA and proteins in input, and IP were analyzed by Western blotting using the indicated Abs. (D) OGT interacts with the A domain of HIRA. GFP-tagged full length or different domains of HIRA (A, AB, and C, Upper) were expressed in 293T cells. Total lysates were subject to IP with antibodies against GFP, followed by Western blotting using the indicated Abs. White arrows indicates GFP-tagged full length or different domains of HIRA. (E) UBN1 mediates the OGT–HIRA interactions. GFP-tagged, full-length UBN1 or a UBN1 mutant deficient in HIRA binding was expressed in 293T cells and immunoprecipitated by antibodies against GFP. (F) OGT interacts with UBN1 in vitro. Full-length HIS-HIRA and Flag-UBN1 were expressed alone or in combination in Sf9 insect cells and were tested for the ability to bind recombinant GST-OGT. GST-OGT bound proteins were pulled down and were analyzed by Western blotting using the indicated antibodies. GST-REGα, a mammalian proteasome binding protein, was used as a negative control.