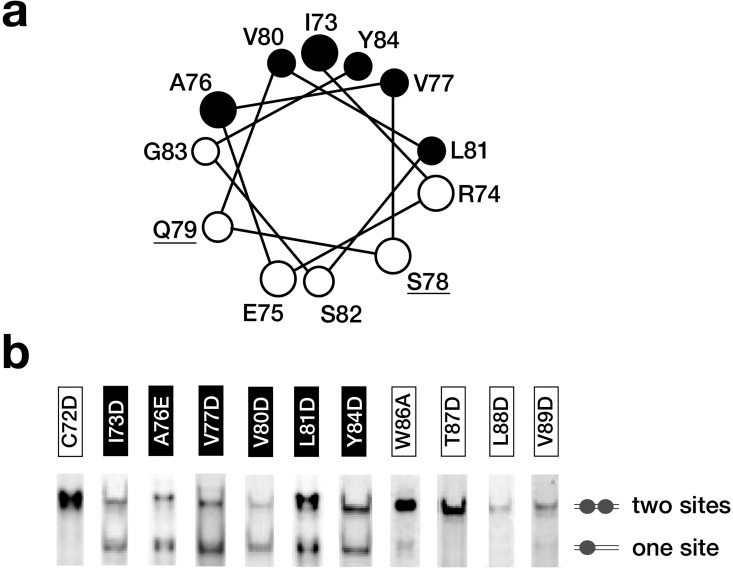
Fig 3. Nonradioactive EMSA assessment of substitution mutants in the dimerization region.
(a) The dimerization region sequence was placed on a helical wheel with hydrophobic amino acids marked black and hydrophilic amino acids marked white to show the amphipathic nature of the predicted helix. S78 and Q79 (underlined) were substituted with alanine in a previous study with no effect on dimerization [25]. (b) For each substitution mutant presented in Fig 1, an EMSA was performed with a CC36 double site DNA probe and a substoichiometric protein to DNA ratio. Mutants with a nonfunctional dimerization domain are observed as a mixture of one site and two-site occupancies while mutants that retain a functional dimerization domain are observed exclusively as a two site occupancy. Mutants that do not have a functional dimerization domain from this qualitative assay coincide with the hydrophobic amino acids on the helical wheel.