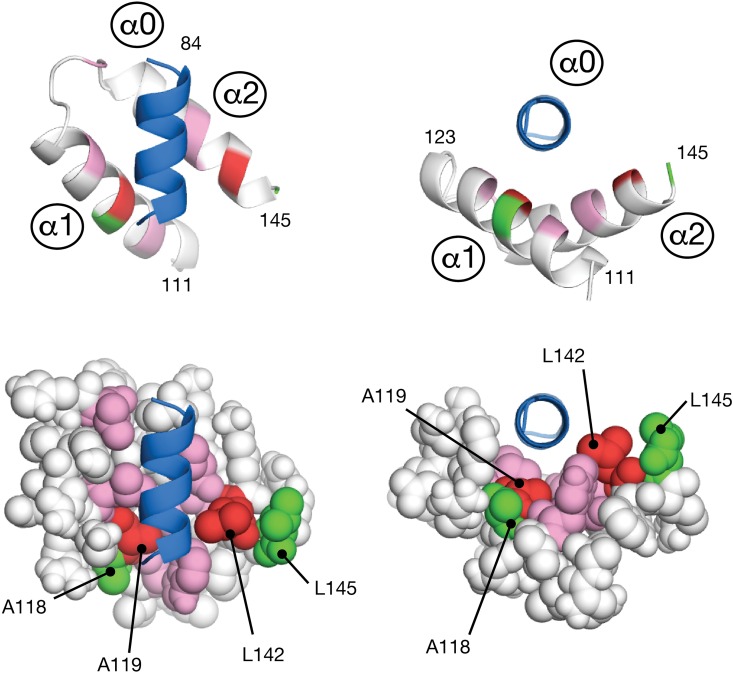
Fig 5. Molecular docking of a potential dimerization region helix upon the SOX9 HMG domain.
Guided by substitution mutagenesis data, the dimerization region was modeled as one amphipathic helix (α0; blue), and docked onto a cleft formed by α1 and α2 of the HMG domain. Following the color scheme in Fig 4, amino acids that prevented and retained dimerization are red and green, respectively. Additional amino acids colored pink (W115, L123, L130, T138, L139, and T146) were include with A119 and L142 to form a contingous hydrophobic surface for peptide docking.