Abstract
Background and study aims: Colorectal endoscopic submucosal dissection (ESD) is an attractive method for en bloc resection of larger flat neoplastic lesions. Experience with this method is limited in the Western World.
Patients and methods: A total of 182 consecutive flat or sessile colorectal lesions (cecum n = 43; right-sided colon n = 65; left-sided colon n = 11, rectum: n = 63) with a size > 20 mm (mean 41.0 ± 17.4 mm) were resected in 178 patients. The data were recorded prospectively.
Results: ESD was technically feasible in 85.2 % of patients with a mean procedure time of 127.5 min (± 99.8) min and a complication rate of 11.5 % (microperforation 9.3 %, delayed bleeding 2.7 %, no case of emergency surgery, 30-day mortality rate 0 %). For 155 successfully completed procedures the en bloc and R0 resection rates were 88.4 and 62.6 %. Efficacy was better for smaller lesions (20 mm to 49 mm; n = 131) than for larger lesions (50 mm to 140 mm; n = 51) with R0 rates of 70.8 vs. 40.5 % (P < 0.001) and procedure times of 92.7 ± 62.4 minutes vs. 217.0 ± 120.9 minutes (P < 0,001).
Conclusions: This series confirms the efficacy of ESD for en bloc resection of colorectal lesions > 20 mm. Results are satisfactory for lesions up to 50 mm. ESD for larger lesions was associated with low R0 resection rates and very long procedure times. The clinical consequences of microperforations were minor and do not argue against the spread of ESD in the West.
Meeting presentations: The data were presented in part at DDW 2014, Chicago IL, USA (Gastrointest Endosc 2014; 79: AB536)
Introduction
Incomplete adenoma resections are not uncommon and are estimated to account for up to one fifth of interval cancers 1 2. Currently, endoscopic mucosal resection is the standard for the treatment of flat or sessile lesions in the Western World 3. However, lesions larger than 20 mm usually cannot be removed en bloc by EMR and are then resected in fragmented fashion as endoscopic piecemeal mucosal resection (EPMR) with reported recurrence in up to one third of the cases 4 5. In contrast, endoscopic submucosal dissection (ESD) allows en bloc resection of flat or sessile colorectal lesions larger than 20 mm and has become the standard of practice in Japan 6. The superiority of ESD over EPMR with regard to complete resection and lower recurrence rates has been demonstrated in several meta-analyses 7 8. ESD even allows en bloc resection of lesions exceeding 5 cm 9 10 11 and – because it is less invasive – may be superior to transanal endoscopic microsurgery 12 13 or laparoscopic assisted colon resection 14 15.
Although colorectal ESD is an attractive method for resection of larger flat or sessile lesions, several disadvantages have hampered its spread in the Western World. Thus, access to a colorectal lesion can be technically demanding and time-consuming, in particular in the proximal colon and within the flexures. Moreover, because the colonic wall is thin, the method is potentially associated with a higher complication rate than EPMR. Last, but not least, training opportunities for ESD in the Western countries are rare 16 17. Consequently, experience with colorectal ESD in the West is limited and studies have mainly focused on the treatment of rectal lesions 18 19 20 21 22 23 24 25 26.
Here, we report prospectively recorded observational data on colorectal ESD in 182 lesions in 178 patients with the majority of lesions localized proximal to the rectum.
Patients and methods
Patients and lesions
Between September 2012 and October 2015 we performed 182 consecutive ESD procedures on 178 patients (male/female: 105/73; median age 70 years, range 46 – 92). Inclusion criteria were informed consent, age > 18 years, sessile or laterally spreading adenomatous lesion > 20 mm. Exclusion criteria were coagulopathy (international normalized ratio [INR] > 1.5; thrombocytopenia < 100 g/L), dual platelet inhibitor therapy or oral anticoagulation that could not be interrupted, pregnancy and lactation, signs of submucosal tumor invasion, life expectancy < 6 months. Data on ESD procedures were analyzed from a prospectively recorded database (ClinicWinData, E&L, Erlangen, Germany). The study was approved by the Ethics Committee of the University of Bonn (registration number 35613) and informed consent was obtained from all patients.
ESD training
Procedures were carried out by a single endoscopist (F. L. D.) who had received training on animal ex vivo models, life pig models, and tutorials by Japanese experts. The training included 2 single-day ESD workshops on ex vivo models (Olympus Medical, Germany), a 2-day training course with life pig models and 7 2-day tutorials with the Japanese Experts Tsuneo Oyama, Akiko Takahashi, Toshio Uraoka and Naohisa Yahagi (Workshop on ESD Expert Training and ESD Clinical Tutoring organized by Frieder Berr, Paracelsus Medical University Salzburg, Austria). In addition, multiple ESD procedures were observed during 2 visits to Japan with Tsuneo Oyama (Saku Central Hospital Advanced Care Center, Nagano) and Naohisa Yahagi (Keio University School of Medicine).
ESD procedures
ESD procedures were carried out under conscious sedation with propofol (B Braun Melsungen, Germany) and midazolam (Roche Pharma, Grenzach-Whylen, Germany); some rectal procedures were performed without sedation. The equipment included standard endoscopes fitted with a 3-mm transparent hood (D-201-12704/D-201-15004), an irrigation pump (OFP-2) and insufflation of carbon dioxide (UCR device; all from Olympus Medical Systems, Tokyo, Japan). Gastroscopes (GIF 1-TQ160, GIF-HQ190) were used for lesions confined to the rectum and distal colon and standard or pediatric colonoscopes (CF-H180 AL, CF-HQ190, PCF 180 AL; all from Olympus Medical Systems, Tokyo, Japan) for more proximal lesions. After detailed endoscopic evaluation and marking of the lesion, the submucosa was injected with glycerol (Glyceol Solution; Chugai Pharmaceutical Co. Ltd., Tokyo, Japan; procedures #1 – 52) or gelatin solution (Gelafundin 4 %; B Braun Melsungen, Melsungen, Germany; procedures #53 – 182) and indigo carmine 0.01 % (Novaplus, Lake Forrest, IL, USA) using a 25 G injector needle (NM-400U-0525). ESD was carried out with dual knife (n = 127), sequential use of dual knife/hook knife (n = 31) or hook knife (n = 24) and a hemostatic forceps (KD-560U, KD-120UR, FD-410LR; all from Olympus) (Fig. 1). A hook knife was preferred in cases when access to the lesion was difficult or dense fibrosis was observed. There was no difference with respect to the perforation rate. The settings of the VAIO 200S electrosurgical unit (Erbe Elektromedizin, Tübingen, Germany) were “soft coagulation” (effect 5/50 W) for initial marking of the target lesion, “EndoCut Q” (effect 2, time 3, interval 3) for mucosal incision and “forced coagulation” (effect 3/30 – 40 W) for submucosal dissection or occlusion of larger vessels or bleeding spots with the hemostatic forceps. To prevent delayed bleeding, careful additional coagulation and/or hemoclips were used at the end of preparation. Clipping was also performed to close any suspected or visible microperforation (EZ clip, Olympus; Instinct Clip, Cook Medical, Mönchengladbach, Germany).
Fig. 1.
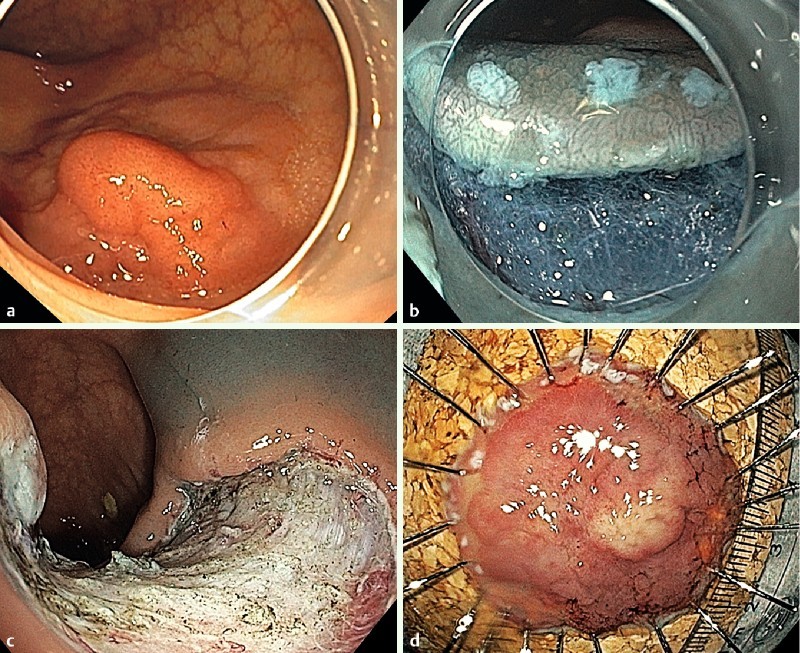
a ESD of a high grade IEN in the ascending colon. Aspect of the lesion (0-IIa/0-Is; LST-granular nodular). b Initial incision of the mucosal layer and submucosal dissection. Note the marking dots. c Aspect of the resection site. d Specimen pinned on corkboard.
Histopathology
The specimens were pinned on a corkboard and fixed in 4 % phosphate buffered formaldehyde. Histopathologic examination was performed with particular care to the lateral and vertical margins in order to confirm a complete resection of the lesion 27. We classified lesions with absence of adenoma/carcinoma tissue in vertical and lateral margins as R0, those with even micro-focal residual adenoma at the coagulation zone as R1 and all piecemeal resections as Rx. Patients with the diagnosis of invasive cancer were discussed in our weekly interdisciplinary tumor board. According to the guidelines of the German Cancer Society surgical resection was recommended for high-risk lesions (R1, submucosal infiltration > 1000 µm, infiltration of lymphatic or blood vessels, poor differentiation G3/4) 28.
Post-procedural care
After the ESD procedure, patients were kept on a clear liquid diet and fed a light meal during the first post-interventional day. Clinical and laboratory controls were carried out 6 hours to 10 hours after the procedure and on the first day after intervention, and as required in case of post-interventional complaints. Most patients with endoscopically treated microperforations received antibiotics for 1 day to 3 days, depending on their clinical course. Delayed bleeding as defined by significant blood loss (> 3 units) was treated with endoscopic hemostasis. The vast majority of patients were discharged from the hospital after 2 days to 3 days. Follow-up endoscopic controls were recommended according to current German guidelines, i. e. after 6 months 28.
Definition of complications
Perforation was assumed if there was clinical evidence during the procedure (i. e. transmural cut). We defined delayed bleeding as significant bleeding (loss of 3 hemoglobin units) after completion of the ESD procedure.
Statistics
Data analysis was done using standard software (Microsoft Excel for Mac 2011/Microsoft and SPSS package version 23.0/IBM). The statistical tests were as follows: Pearson’s and Fisher’s chi-squared test for categorical data and Mann Whitney-U test for comparison of numerical data; univariate and multivariate analysis for exploration of possible associations between complications and patient or lesion characteristics; linear regression analysis for the association between lesion size and procedures time. A P value < 0.05 was considered statistically significant.
Results
Efficacy
An ESD procedure was initiated for 182 consecutive flat or sessile colorectal lesions in 178 patients (cecum n = 43; right-sided colon n = 65; left-sided colon n = 1, rectum n = 63). The mean lesion size was 41.0 mm (± 17.4 mm). ESD was technically feasible in 155/182 (85.2 %) of the interventions with a mean procedure time of 127.5 minutes (± 99.8 minutes). In a total of 27 (14.8 %) procedures, technical difficulties (severe fibrosis, n = 17; lack of appropriate access to the lesion, n = 10) resulted in conversion to EPMR (n = 24; 13.2 %) or to an interruption of the procedure and referral for elective surgery (n = 3; 1.6 %). The en bloc rate for 155 successfully completed procedures was 88.4 % (137/155), the R0 rate 62.6 % (97/155). All specimens classified as R1 resections had a positive lateral margin (most often microfocally) but negative vertical margins.
When stratifying results by lesion size (20 mm to 49 mm; n = 131 vs. 50 mm to 140 mm; n = 51) en bloc resection rates were relatively similar (89.4 vs. 85.7 %) for both groups. However, smaller lesions had higher R0 rates (70.8 vs. 40.5 %, P < 0,001), shorter procedure times (92.7 ± 62.4 vs. 217.0 ± 120.9 minutes, P < 0,001) and a lower complication rate (9.2 % vs. 17.6 %; Table 1). At the time of writing, endoscopic follow-up data are available for 23 of 40 lesions with a median follow-up time of 7 months (range 2.3 months to 31 months) with en bloc but R1 resections and showed recurrence rate of 4.3 % (1/23). Procedure time correlated with the size of the lesion (Fig. 2).
Table 1. Outcome of ESD procedures by size.
| All lesions | Lesion size20 mm – 49 mm | Lesion size50 mm – 140 mm | P value(by size) | |
| All procedures | (n = 182) | (n = 131) | (n = 51) | |
| Lesion size, mean (± SD) | 41.0 mm(± 17.4) | 32.5 mm(± 7.4) | 61.4 mm(± 12.5) | ./. |
| Localization | ||||
| Cecum | 43 (23.6 %) | 30 (22.9 %) | 13 (25.5 %) | n.s. |
| Right-sided colon | 65 (35.7 %) | 51 (38.9 %) | 14 (27.5 %) | n.s. |
| Left-sided colon | 11 (6.0 %) | 9 (6.9 %) | 2 (3.9 %) | n.s. |
| Rectum | 63 (34.6 %) | 41 (31.3 %) | 22 (43.1 %) | n.s. |
| Procedures | ||||
| Procedure time, mean (± SD) | 127.5 min (± 99.8) | 92.7 min(± 62.4) | 217.0 min(± 120.9) | P < 0.001 |
| Conversion to EPMR or surgery | 27 (14.8 %) | 18 (13.7 %) | 9 (17.6 %) | n.s. |
| Complications | 21 (11.5 %) | 12 (9.2 %) | 9 (17.6 %) | n.s. |
| Perforation | 17 (9.3 %) | 11 (8.4 %) | 6 (11.8 %) | n.s. |
| Delayed bleeding | 5 (2.7 %) | 2 (1.5 %) | 3 (5.9 %) | n.s. |
| Histology | ||||
| Carcinoma | 13 (7.1 %) | 10 (7.6 %) | 3 (5.9 %) | n.s. |
| HG-IEN | 48 (26.4 %) | 29 (22.1 %) | 19 (37.2 %) | P < 0.05 |
| LG-IEN | 101 (55.5 %) | 77 (58.8 %) | 24 (47.1 %) | n.s. |
| SSA | 20 (11.0 %) | 15 (11.5 %) | 5 (9.8 %) | n.s. |
| Completed procedures only | (n = 155) | (n = 113) | (n = 42) | |
| En bloc resection | 137 (88.4 %) | 101 (89.4 %) | 36 (85.7 %) | n.s. |
| R0 resection | 97 (62.6 %) | 80 (70.8 %) | 17 (40.5 %) | P < 0.001 |
HG-IEN, high-grade intraepithelial neoplasia; LG-IEN, low-grade intraepithelial neoplasia; SSA, sessile serrated adenoma.
Fig. 2.
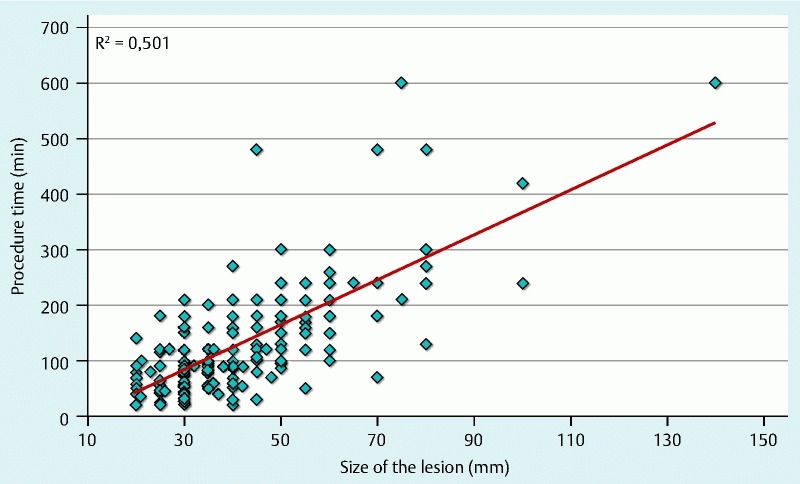
Correlation of ESD procedure times with lesion size. Calculation was done by linear regression analysis.
Complications
Complications were observed in 21/182 procedures (11.5 %). We had 17 (9.3 %) microperforations. In 6 of these lesions, dense fibrosis and a non-lifting sign were observed; none of these lesions harbored invasive cancer. All perforations could be treated conservatively with hemoclips and antibiotics. The clinical course after perforation differed with the localization. Thus, all 6 perforations in the rectum remained asymptomatic with an increase in leucocyte count of more than 2-fold above pretreatment level in only 1 patient and discharge on the second day after intervention for all patients. In contrast, 3/11 patients with perforations localized proximal to the rectum had post-procedural pain, 6 of 11 patients had an increase in leucocyte counts, and the length of hospital stay was longer (median 3.3 days; range 2 – 7) (Table 2). Delayed bleeding was observed in 5/182 of the procedures (2.75 %) and could be treated by endoscopic hemostasis. In 4 of 5 cases of delayed bleeding, the patients were on anticoagulation and/or antiplatelet agents. None of the patients required emergency surgery and the 30-day mortality rate was 0 %. On univariate and multivariate analysis (data not shown), no statistically significant association was seen between complications and age, gender, localization or size of the lesion or the number of performed procedures.
Table 2. Perforation location and outcome.
| LocalizationRectum | Localization proximal to rectum | P value | |
| Number of complications | 6/63 (9.5 %) | 11/119 (9.2 %) | n.s. |
| Age. Median (range) | 73 (52 – 86) | 66 (49 – 86) | n.s. |
| Sex (f/m) | 1/5 | 4/7 | n.s. |
| Fibrosis/non-lifting | 2/6 (33.3 %) | 4/11 (36.4 %) | n.s. |
| Leucocyte increase (-fold). Median (range) | 1.66 (1.07 – 2.31) | 2.30 (1.03 – 3.06) | n.s. |
| Associated post-procedural pain | 0/6 | 3/11 | n.s. |
| Hospital stay. Median (range) | 2 (2 – 2) | 3.3 (3 – 7) | P < 0.05 |
Learning curve
We also compared efficacy and complication rate for the first half (n = 91 consecutive procedures) versus the second half of the ESD procedures. A highly significant difference was observed for en bloc resection rates of rectal lesions (69.6 % vs. 97.5 %; P < 0.001), but not for lesions localized proximal to the rectum. Moreover, there was no statistically significant difference in en bloc, R0 or complications rates between the two groups.
Histopathology
The results of histopathology of all 182 lesions were invasive carcinoma (n = 13; 7.1 % with 4 non-curative R0 resections due to submucosal invasion depths > 1000 µm and/or lymphangio-invasion), high-grade intraepithelial neoplasia (n = 48; 26.4 %), low-grade tubular-villous adenoma (n = 101; 55.5 %) and serrated adenoma (n = 20; 11.0 %). The correlation of lesion characteristics and final results of histopathology is summarized in Table 3.
Table 3. Characteristics of treated lesions and corresponding histology.
| 0-Is(n = 33) | 0-IIa/0-Is(n = 86) | 0-Iia(n = 58) | 0-IIa/0-Iic(n = 5) | LST-G(n = 103) | LST-NG(n = 46) | |
| Size. mean ± SD | 30.4 mm(± 7.6) | 48.7 mm(± 19.4) | 35.9 mm(± 13.6) | 40.0 mm(± 12.7) | 44.0 mm(± 18.9) | 33.8 mm(± 11.5) |
| Localization rectum | 16 (48 %) | 38 (44 %) | 6 (10 %) | 3 (60 %) | 43 (42 %) | 4 (9 %) |
| Histology | ||||||
| SSA | 0 (0 %) | 1 (1 %) | 19 (33 %) | 0 (0 %) | 1 (1 %) | 19 (41 %) |
| LG-IEN | 23 (70 %) | 54 (63 %) | 24 (41 % | 0 (0 %) | 64 (62 %) | 14 (30 %) |
| HG-IEN | 5 (15 %) | 28 (33 %) | 11 (19 %) | 4 (80 %) | 34 (33 %) | 9 (20 %) |
| Carcinoma | 5 (15 %) | 3 (3 %) | 4 (7 %) | 1 (20 %) | 4 (4 %) | 4 (9 %) |
Paris 0-Is lesions were not classified as LST; size is given as mean ± standard deviation.LST-G, laterally spreading tumor granular type; LST-NG, laterally spreading tumor non-granular type; SSA, sessile serrated adenoma; LG-IEN, low-grade intraepithelial neoplasia; HG-IEN, high-grade intraepithelial neoplasia.
Discussion
Colorectal ESD has mainly been established and evaluated in Japan, where it has become the standard treatment for larger neoplastic lesions that need en bloc resection 6. Advantages of ESD include higher en bloc and R0 resection rates (in comparison to EMR) and lower invasiveness (in comparison to laparoscopic surgery). Recent Asian studies with > 500 interventions report en bloc and R0 resection rates of 84 – 94.5 % 11 29 30. European studies are much smaller and mainly focused on ESD for rectal lesions 18 19 20 21 22 23 24 25 26 with lower en bloc (64 % to 90 %) and R0 (53 % to 81 %) resection rates.
In this single-center study, we report 182 colorectal ESD procedures performed on lesions mostly localized proximal to the rectum. We observed a mean procedure time of 127.5 min with en bloc and R0 resection rates of 88.4% and 62.6 %, respectively, and a better rate of efficacy with ESD for smaller (20 mm to 49 mm) than for larger lesions (n = 51) with R0 rates of 70.8 % vs. 40.5 % and procedure times of 92.7 vs. 217.0 minutes, respectively. In the group with lesions up to 49 mm, the efficacy is in line with data from the early period of colorectal ESD in Asia 31 and within the upper range of European reports 18 19 20 21 22 23 24 25 26. In line with data on larger colorectal lesions 9 10, we found that lesions larger than 50 mm resulted in a significantly lower R0 rate (40.5 %) and longer mean procedure time. Although these data apparently argue against ESD for larger colorectal lesions, the en bloc rate in our series was still similar to the results for treatment of smaller lesions (85.7 %) and so far, we have observed a low recurrence rate after R1 resections (4.5 %). The complication rate of 11.5 % is also within the reported range 6 18 19 20 21 22 23 24 25 26 with a trend to higher perforation rates for ESD of larger lesions. Although we could not identify a single significant risk factor in this series, the higher perforation rate will probably be the consequence of risk factors reported in previous studies 32 33, in particular larger lesion size and the relatively higher number of lesions localized in the right-sided colon. However, all perforations could be treated endoscopically and had a relatively mild clinical course, particularly if they were localized in the rectum. Emergency surgery after ESD has been reported to be around 1 % 7; in the current study, we had no case of emergency surgery and the 30-day mortality rate was 0 %. Thus, although the perforation rate for colorectal ESD is higher than for that for EPMR, its clinical relevance is relatively minor. The rate of delayed bleeding is also comparable to recent Asian publications 6. It probably reflects the patients´ comorbidity since 4 out of 5 patients with bleeding were being treated with anticoagulants and/or antiplatelet agents. Finally, we did observe a significant increase in the en bloc resection rate for rectal lesions. However, a learning curve – which has been reported in many 20 23 24 34 but not all published studies 22 – was not observed for lesions localized proximal to the rectum, and we did not observe a decrease in complication rate. These findings probably reflect the heterogeneous group of treated lesions with different localizations and lesions sizes.
While the study presented here comprises the largest number of colorectal ESDs reported from Europe and also includes lesions located proximal to the rectum, there are several limitations. Thus, the study was conducted in a single-center, single-operator design and it is therefore difficult to generalize the reported results. Moreover, the study has a retrospective design and lacks a control group (e. g. with EPMR). Nevertheless, the data should give some insight into establishing colorectal ESD under the conditions of the Western world (e. g. lack of sufficient training with gastric lesions) and the results of this study are in fact similar to reports from Asia on learning colorectal ESD without prior ample experience with gastric ESD 34 35.
Conclusions
In summary, the data presented confirm the efficacy of ESD for endoscopic en bloc resection of colorectal lesions > 20 mm. Efficacy is satisfactory for lesions up to 50 mm, but ESD for larger lesions was associated with low R0 resection rates and very long procedure times. Therefore, alternative methods such as either EPMR (short procedure time) or laparoscopic resection (higher R0 rate) should still be considered for such cases. Although the perforation rate is higher than that reported for EPMR, the clinical consequences of these microperforations were minor and should not argue against the spread of ESD in the West.
Acknowledgements
We gratefully acknowledge our fellow gastroenterologists for patient referrals and particularly the Endoscopy Nursing Team of Gemeinschaftskrankenhaus Bonn for their enduring support. We are also indebted to Professor Gerhard Kleber (Aalen, Germany) and to Professor Frieder Berr (Paracelsus Medical University, Salzburg, Austria) for initiating and maintaining interest in colorectal ESD and to Professor Tilman Sauerbruch for helpful comments on the manuscript.
Footnotes
Competing interests: None
References
- 1.Pohl H, Srivastava A, Bensen S P. et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–800. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Robertson D J, Lieberman D A, Winawer S J. et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949–956. doi: 10.1136/gutjnl-2012-303796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang J H, Konda V, Abu Dayyeh B K. et al. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82:215–226. doi: 10.1016/j.gie.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Belderbos T D, Leenders M, Moons L M. et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388–402. doi: 10.1055/s-0034-1364970. [DOI] [PubMed] [Google Scholar]
- 5.Knabe M, Pohl J, Gerges C. et al. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109:183–189. doi: 10.1038/ajg.2013.419. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka S, Kashida H, Saito Y. et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417–434. doi: 10.1111/den.12456. [DOI] [PubMed] [Google Scholar]
- 7.Repici A, Hassan C, De Paula Pessoa D. et al. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy. 2012;44:137–150. doi: 10.1055/s-0031-1291448. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Zhang X H, Ge J. et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: a meta-analysis. World J Gastroenterol. 2014;20:8282–8287. doi: 10.3748/wjg.v20.i25.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi Y, Shinozaki S, Sunada K. et al. Efficacy and safety of endoscopic submucosal dissection for superficial colorectal tumors more than 50 mm in diameter. Gastrointest Endosc. 2016;83:602–607. doi: 10.1016/j.gie.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Jung da H, Youn Y H, Kim J H. et al. Endoscopic submucosal dissection for colorectal lateral spreading tumors larger than 10 cm: is it feasible? Gastrointest Endosc. 2015;81:614–620. doi: 10.1016/j.gie.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka S, Toyonaga T, Morita Y. et al. Feasibility and Safety of Endoscopic Submucosal Dissection for Large Colorectal Tumors. Surg Laparosc Endosc Percutan Tech. 2015 doi: 10.1097/SLE.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 12.Arezzo A, Passera R, Saito Y. et al. Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc. 2014;28:427–438. doi: 10.1007/s00464-013-3238-3. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguti F S, Nahas C S, Marques C F. et al. Endoscopic submucosal dissection versus transanal endoscopic microsurgery for the treatment of early rectal cancer. Surg Endosc. 2014;28:1173–1179. doi: 10.1007/s00464-013-3302-z. [DOI] [PubMed] [Google Scholar]
- 14.Kiriyama S, Saito Y, Yamamoto S. et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy. 2012;44:1024–1030. doi: 10.1055/s-0032-1310259. [DOI] [PubMed] [Google Scholar]
- 15.Ahlenstiel G, Hourigan L F, Brown G. et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014;80:668–676. doi: 10.1016/j.gie.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Berr F, Ponchon T, Neureiter D. et al. Experimental endoscopic submucosal dissection training in a porcine model: learning experience of skilled Western endoscopists. Dig Endosc. 2011;23:281–289. doi: 10.1111/j.1443-1661.2011.01129.x. [DOI] [PubMed] [Google Scholar]
- 17.Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in western countries? J Gastroenterol Hepatol. 2013;28:406–414. doi: 10.1111/jgh.12099. [DOI] [PubMed] [Google Scholar]
- 18.Hurlstone D P, Atkinson R, Sanders D S. et al. Achieving R0 resection in the colorectum using endoscopic submucosal dissection. Br J Surg. 2007;94:1536–1542. doi: 10.1002/bjs.5720. [DOI] [PubMed] [Google Scholar]
- 19.Farhat S, Chaussade S, Ponchon T. et al. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43:664–670. doi: 10.1055/s-0030-1256413. [DOI] [PubMed] [Google Scholar]
- 20.Probst A, Golger D, Anthuber M. et al. Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy. 2012;44:660–667. doi: 10.1055/s-0032-1309403. [DOI] [PubMed] [Google Scholar]
- 21.Repici A, Hassan C, Pagano N. et al. High efficacy of endoscopic submucosal dissection for rectal laterally spreading tumors larger than 3 cm. Gastrointest Endosc. 2013;77:96–101. doi: 10.1016/j.gie.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 22.Agapov M, Dvoinikova E. Factors predicting clinical outcomes of endoscopic submucosal dissection in the rectum and sigmoid colon during the learning curve. Endosc Int Open. 2014;2:E235–240. doi: 10.1055/s-0034-1377613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berr F, Wagner A, Kiesslich T. et al. Untutored learning curve to establish endoscopic submucosal dissection on competence level. Digestion. 2014;89:184–193. doi: 10.1159/000357805. [DOI] [PubMed] [Google Scholar]
- 24.Bialek A, Pertkiewicz J, Karpinska K. et al. Treatment of large colorectal neoplasms by endoscopic submucosal dissection: a European single-center study. Eur J Gastroenterol Hepatol. 2014;26:607–615. doi: 10.1097/MEG.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 25.Rahmi G, Hotayt B, Chaussade S. et al. Endoscopic submucosal dissection for superficial rectal tumors: prospective evaluation in France. Endoscopy. 2014;46:670–676. doi: 10.1055/s-0034-1365810. [DOI] [PubMed] [Google Scholar]
- 26.Spychalski M, Zelga P, Dziki A. Key factors in achieving successful endoscopic dissection of rectal tumors: early results of 33 consecutive rectal endoscopic submucosal dissections in Polish academic center. Surg Laparosc Endosc Percutan Tech. 2015;25:173–177. doi: 10.1097/SLE.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 27.Nagata K, Shimizu M. Pathological evaluation of gastrointestinal endoscopic submucosal dissection materials based on Japanese guidelines. World J Gastrointest Endosc. 2012;4:489–499. doi: 10.4253/wjge.v4.i11.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pox C, Aretz S, Bischoff S C. et al. [S3-guideline colorectal cancer version 1.0] Z Gastroenterol. 2013;51:753–854. doi: 10.1055/s-0033-1350264. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Uraoka T, Yamaguchi Y. et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Lee E J, Lee J B, Lee S H. et al. Endoscopic submucosal dissection for colorectal tumors--1,000 colorectal ESD cases: one specialized institute's experiences. Surg Endosc. 2013;27:31–39. doi: 10.1007/s00464-012-2403-4. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Oka S, Kaneko I. et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100–107. doi: 10.1016/j.gie.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 32.Sato K, Ito S, Kitagawa T. et al. Factors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumors. Surg Endosc. 2014;28:2959–2965. doi: 10.1007/s00464-014-3558-y. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi Y, Iishi H, Tanaka S. et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis. 2014;29:1275–1284. doi: 10.1007/s00384-014-1947-2. [DOI] [PubMed] [Google Scholar]
- 34.Shiga H, Kuroha M, Endo K. et al. Colorectal endoscopic submucosal dissection (ESD) performed by experienced endoscopists with limited experience in gastric ESD. Int J Colorectal Dis. 2015;30:1645–1652. doi: 10.1007/s00384-015-2334-3. [DOI] [PubMed] [Google Scholar]
- 35.Yang D H, Jeong G H, Song Y. et al. The feasibility of performing colorectal endoscopic submucosal dissection without previous experience in performing gastric endoscopic submucosal dissection. Dig Dis Sci. 2015;60:3431–3441. doi: 10.1007/s10620-015-3755-0. [DOI] [PubMed] [Google Scholar]


