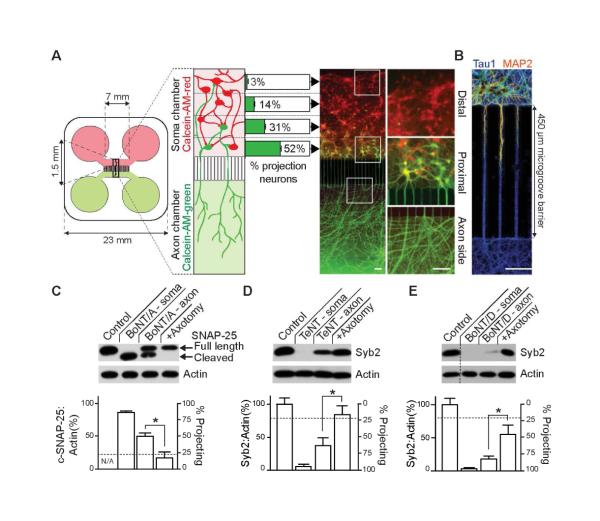
Figure 1. CNTs cleave substrates beyond the levels predicted by connectivity of neurons in compartmentalized microfluidic devices.
(A) Dissociated hippocampal neurons were seeded into the soma chamber (top, red) of a microfluidic device. The number of cells that extend axons through the microchannels to the axon chamber (projecting cells) was determined by differential Calcein-AM-red and -green staining, added to the soma (top) and axon (bottom) chambers, respectively. Projecting cells are green/yellow, non-projecting cells are red. The number of projecting neurons drops sharply from 52 ± 2.9% of cells residing proximal to the microchannels in the soma side, to 31 ± 3.5 % in the mid-proximal, 14 ± 2.5 % in mid-distal, and to just 3 ± 0.6 % in the most distal region. Representative image of Calcein-stained neurons; white boxes denote regions that are magnified in the right panel. (B) Dendrites fail to reach the axon side due to the length of the microgroove barrier, creating an isolated axonal compartment, as illustrated by staining with MAP2, to mark dendrites (red/orange) and tau1, to mark axons (blue). (C) BoNT/A was added to the soma (10 nM) or axon (30 nM) side of the microfluidic device. After 48 hours, cells in the soma chamber were assayed for substrate cleavage via immunoblot analysis. The horizontal dotted line represents the total fraction of cells projecting to the axon side (24% ± 1.5%). Cleavage of substrate was abolished by axotomy. Similarly, TeNT (D), or BoNT/D (E) were added to either the soma or axon side of microfluidic devices, and cleavage of syb2 was assayed by immunoblot analysis. All plotted values are averages ± SEM; experiments were carried out with 3-5 independent rat litters. Scale bars: 100 μm. See also Figures S1,2.