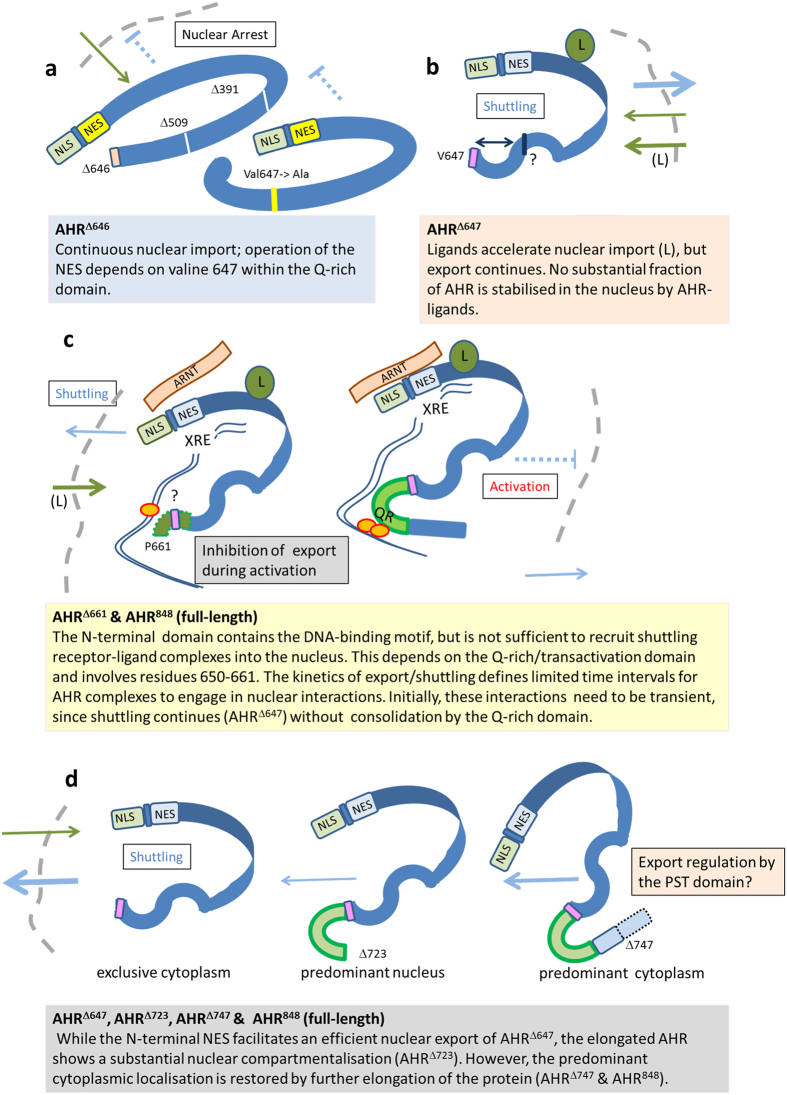
Figure 5. Nuclear export and intracellular trafficking of the human AHR are regulated by defined motifs.
(a) The nuclear localisation signal (NLS) within the N-terminal domain triggers continuous basal import (green arrows) into the nucleus (shuttling). Contrary to this autonomous import mechanism, function of the adjacent nuclear export signal (NES) depends on C-terminal motifs, especially the mandatory residue V647. (b) Ligands (marked with L) accelerate import, while continued export (blue arrow) counteracts nuclear sequestration of the AHR, thus maintaining a predominant cytoplasmic fraction that is receptive for interactions with ligands. Notably, mutants that lack parts of the C-terminal domain (AHRΔ647 and AHRΔ650) do not efficiently accumulate in the nucleus, although nuclear transfer is accelerated by ligands. (c) Export of the AHR continues in the presence of ligands. Activation of the AHR might involve several passages of receptor molecules that need to engage in further associations with nuclear components during limited time intervals. Stable associations of the AHR with the nucleus likely require a defined section of the Q-rich domain (green, Pro 661 is indicated). However, it is as yet completely unknown how this motif stabilises nuclear compartmentalisation or whether it promotes interactions of the transactivation domain with transcription factors. (d) The N-terminal NES and the V647 motif facilitate an efficient nuclear export of AHRΔ647, leading to a nearly exclusive cytoplasmic pattern. On the other side, the full-length AHR contains an additional motif within the PST domain to maintain a predominantly cytoplasmic compartmentalisation.