Abstract
Background
For women at potentially increased risk for ovarian cancer, data regarding screening and risk reduction are limited. Previous studies have reported on the behaviors of BRCA mutation carriers, but less is known about the behaviors of non-BRCA carriers. We surveyed a large cohort of women after BRCA testing to identify the prevalence and posttest predictors of risk-reducing and screening interventions.
Methods
A median of 3.7 years after BRCA testing, 1447 women who received genetic counseling and BRCA testing at 2 hospital sites were surveyed, with a 77.6% response rate. We analyzed data from 1077 survey respondents. We performed univariate and multivariate logistic regression analyses to identify predictors of risk-reducing salpingo-oophorectomy (RRSO), screening transvaginal ultrasonography (TVUS), and screening serum cancer antigen 125 (CA-125).
Results
Among the respondents, 201 women (18.7%) received positive test results for a deleterious mutation, 103 women (9.6%) received true-negative results, and 773 women (71.8%) received uninformative results. Overall, 19.1% of eligible women underwent RRSO and 39.6% used screening procedures. A positive BRCA result predicted RRSO (odds ratio [OR], 28.1; 95% CI, 16.2-48.6), TVUS (9.5 [4.3-21.0]), and serum CA-125 (13.0 [5.5-29.0]). Similarly, a true-negative BRCA result reduced the OR for RRSO (0.1 [0.0-0.6]), TVUS (0.2 [0.1-0.5]), and serum CA-125 (0.3 [0.1-0.7]). Of the 71.8% of women who received uninformative results after BRCA testing, 12.3% subsequently underwent RRSO, 33.8% reported ever having undergone screening serum CA-125 since BRCA testing, and 37.3% reported ever having undergone screening TVUS since BRCA testing.
Conclusions
Results of BRCA testing strongly predict RRSO and ovarian cancer screening. Use of RRSO and ovarian screening was reported in a sizable percentage of non-BRCA carriers despite insufficient data to determine the effectiveness of these interventions.
Ovarian Cancer is The leading cause of death from gynecologic malignant neoplasms in the developed world, with an estimated 21 880 new cases diagnosed in the United States in 2010 and 13 850 predicted deaths.1 The lifetime risk of developing ovarian cancer is only 1% to 2% in the general population; however, women with deleterious BRCA mutations have a cumulative lifetime risk of developing ovarian cancer of approximately 40% in BRCA1 carriers and approximately 20% in BRCA2 carriers.2,3 In light of these statistics, there has been significant interest in defining the role of ovarian cancer screening in individuals who might be at higher-than-average risk.
There is growing evidence that BRCA carriers who undergo risk-reducing salpingo-oophorectomy (RRSO) significantly reduce ovarian cancer and breast cancer risk, ovarian cancer–related mortality, and even all-cause mortality.4,5 Studies have reported rates of RRSO in BRCA carriers ranging from 12% to 78%. Some researchers have examined the time to RRSO in these women; multiple studies6-11 have shown a median time of 6 months from learning of BRCA-positive results to RRSO in BRCA carriers.
For female BRCA carriers who choose to forego or delay this risk-reducing surgery, recommendations for ovarian cancer screening are conflicting and ambiguous (Table 1). Furthermore, for the vast majority of women who receive uninformative BRCA test results, no guidelines exist.
Table 1. Recommendations for Ovarian Cancer Screening in High-Risk Women.
| Organization | Recommendation |
|---|---|
| American Cancer Society | Women may be screened, but it is not known how helpful the screening tests are.12 |
| American College of Obstetricians and Gynecologists | If appropriate, these women may be offered ovarian cancer screening. Screening with CA-125 measurement and TVUS every 6 mo has been recommended by the National Comprehensive Cancer Network, although evidence is insufficient to demonstrate that current screening methods improve survival rates for these women.13 |
| Canadian Task Force on Preventive Health Care | Insufficient evidence to recommend for or against screening, but expert opinion suggests that these women be referred to an academic health center for regular combination screening.14 |
| National Comprehensive Cancer Network | Screen with TVUS and CA-125 every 6 mo starting at age 35 y or 5-10 y before the youngest relative received an ovarian cancer diagnosis.15 |
| United States Preventive Services Task Force | The positive predictive value of an initially positive screening test would be more favorable for women at higher risk; if ongoing clinical trials show that screening has a beneficial effect on mortality rates, then women at higher risk are likely to experience the greatest benefit.16 |
Abbreviations: CA-125, cancer antigen 125; TVUS, transvaginal ultrasonography.
A substantial body of research has shown that current screening modalities—principally the serum cancer antigen 125 (CA-125) test and transvaginal ultrasonography (TVUS)—have poor diagnostic test characteristics in an average-risk population, with a positive predictive value anywhere from 1.5% for CA-125 and TVUS combined to 14.0% for TVUS alone.17,18 Furthermore, these tests may not improve mortality and may actually lead to significant harm.17,19,20 For example, of 3285 women with false-positive results of ovarian cancer screening in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, 1080 underwent surgical follow-up and 15.0% of these experienced at least 1 serious complication.19
In a population of higher-risk women, several small studies have examined the usefulness of regular CA-125 and TVUS surveillance and found that the positive predictive value of these tests was not significantly improved and that most screening-detected cancers were in advanced stages at the time of detection.17,21-24 Although guidelines13 from several organizations suggest that routine screening TVUS and/or serum CA-125 testing may be offered to high-risk women, evidence is insufficient to demonstrate that these tests provide a survival benefit.
Literature regarding risk-reducing and screening interventions after BRCA testing has been limited to small populations, has reported short follow-up, or has not differentiated the impact of BRCA-positive, BRCA-negative, or uninformative test results. In this study, we expanded on previous work7-10 by reporting on a cohort of 1077 women at risk for hereditary breast and ovarian cancer syndrome who received BRCA testing and were surveyed a median of 3.7 years after genetic testing. We classified ovarian cancer risk-reducing and screening interventions on the basis of BRCA results. Our goals were to describe and compare the rate of RRSO and ovarian cancer screening by BRCA status and to confirm predictors of RRSO and ovarian cancer screening in a large and diverse population with varying levels of ovarian cancer risk.
Methods
Patients
Participants were recruited from the University of California, San Francisco (UCSF) Cancer Risk Program, which provides genetic counseling and BRCA testing at 2 locations: the Helen Diller Family Comprehensive Cancer Center (Diller), a tertiary referral cancer center, and San Francisco General Hospital (SFGH), a public county hospital. Both locations use the same genetic counselors and BRCA testing protocol, in which women are typically eligible for testing if their prior probability of carrying a BRCA mutation is estimated to be at least 5% as calculated using BRCAPRO software (BayesMendel Lab).25
More than 95% of patients who undergo BRCA testing through the Cancer Risk Program agree to an institutional review board–approved follow-up protocol, details of which have been published elsewhere.26 Women tested for BRCA mutations at either UCSF testing site between January 1996 and March 2008 and who were living in the United States in 2008 were considered eligible for this survey. Informed consent was obtained from all participants.
Survey Methods
A comprehensive 22-page survey was designed by method and content experts to examine risk-reducing and screening interventions in all women who received BRCA testing within the UCSF Cancer Risk Program. The survey consisted of a 16-page follow-up module for all participants and a 6-page cancer module for participants who reported any prior cancer diagnosis. Average survey completion time was 20 minutes. We mailed the survey to all women eligible for BRCA testing from both testing sites in 2008. Participants at SFGH were offered the option of completing the survey by telephone or in person in their preferred language (Spanish, Russian, Cantonese, or Mandarin), since 30% of the SFGH population communicates in a language other than English compared with less than 3% of the Diller population.
Measurements
BRCA Test Results
Test results were categorized as either BRCA1/2 mutation positive, true-negative, uninformative negative, or variant of undetermined significance. Positive results occurred when a participant was shown to carry a known deleterious BRCA mutation. True-negative results occurred when a participant received negative test results for a known deleterious family BRCA mutation. Uninformative negative results occurred when a participant received negative BRCA test results without a known family mutation. Variant of undetermined significance results occurred when a participant was found to have a change in DNA that has unknown effects on BRCA protein function. For analyses, participants with variant of undetermined significance results were grouped with participants with uninformative negative results into a single group of uninformative results.
Sociodemographic and Clinical Data
To assess socioeconomic status, we enlisted a third-party company (Nielsen Claritas) to determine income-producing assets for each participant. Nielsen Claritas was provided with anonymized census demographic data to estimate income-producing assets per individual household using several variables, including income and home ownership.27
We collected demographic characteristics, basic medical history, and surgical history. Cancer-specific medical history detailed previous treatments and prior use of risk-reducing and screening measures for breast, ovarian, skin, and colon cancer. Time since testing was calculated as the interval between the date of receiving BRCA test results, verified by medical record, and the date of survey completion.
Risk-Reducing Surgery and Screening Test Use
To assess for RRSO, participants were asked, “Have you undergone surgery at any time to prevent ovarian cancer (prophylactic oophorectomy)?” If they answered yes, follow-up questions included the dates of surgery and whether the fallopian tubes and/or uterus were removed. For participants whose reason for salpingo-oophorectomy was unclear, medical records were reviewed individually and categorized appropriately.
To query screening test use, participants were asked, “Have you ever had a screening CA-125 blood test for ovarian (or primary peritoneal) cancer screening?” A similar question was asked regarding TVUS. For both CA-125 and TVUS, participants were asked to report whether they had received the test in the past year and approximately how many tests they had received in the past 3 years.
Statistical Analysis
The χ2 and standard 2-tailed unpaired t tests were used to identify univariate predictors of RRSO after BRCA testing and ovarian cancer screening within the past year. Because ovarian cancer screening can be a recurrent event, we tallied the number of TVUS and CA-125 screenings by participant in the prior 3 years. Significant variables in univariate analyses were considered for multivariate logistic regression models, specifically, variables initially significant at P < .20 and with proportions large enough to produce stable model estimates. P values estimated from pairwise comparisons with a reference group and group tests of heterogeneity were presented. All analyses were carried out with commercial software (SAS, version 9.2; SAS Institute Inc).
Results
Population Characteristics
Among the 1447 eligible participants, 1123 women (77.6%) responded to the survey. Respondents did not differ significantly from nonrespondents in age, race, BRCA test result, cancer history, or year of BRCA testing (data not shown). The mean (SD) age of participants at the time of the survey was 53 (11) years (Table 2), with a median time since testing of 3.7 years. Forty-six respondents reported undergoing RRSO before their BRCA testing date and were excluded from analyses, leaving 1077 respondents in the study population.
Table 2. Characteristics of 1077 High-Risk Women Who Underwent BRCA Testing.
| No. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Overall (N = 1077) | True-Negative (n = 103) | Uninformative (n = 773) | BRCA Positive (n = 201) |
| Demographics | ||||
| Age at survey, mean (SD), y | 52.9 (11.2) | 49.9 (13.5) | 54.3 (10.7) | 49.5 (10.7) |
| <40 | 120 (11.1) | 19 (18.4) | 58 (7.5) | 43 (21.4) |
| 40-49 | 299 (27.8) | 39 (37.9) | 197 (25.5) | 63 (31.3) |
| 50-59 | 357 (33.1) | 21 (20.4) | 280 (36.2) | 56 (27.9) |
| ≥60 | 301 (27.9) | 24 (23.3) | 238 (30.8) | 39 (19.4) |
| White race | 901 (83.7) | 96 (93.2) | 641 (83.0) | 164 (81.6) |
| Ashkenazi Jewish | 416 (38.7) | 47 (45.6) | 274 (35.5) | 95 (47.3) |
| Socioeconomic status | ||||
| Income-producing assets, $ | ||||
| ≤100 000 | 196 (18.2) | 19 (18.4) | 139 (18.0) | 38 (19.0) |
| 100 001-500 000 | 357 (33.2) | 36 (35.0) | 237 (30.7) | 84 (42.0) |
| 500 001-1 000 000 | 148 (13.8) | 13 (12.6) | 109 (14.1) | 26 (13.0) |
| ≥1 000 001 | 375 (34.9) | 35 (34.0) | 288 (37.3) | 52 (26.0) |
| Testing site | ||||
| SFGH | 74 (6.9) | 2 (1.9) | 63 (8.2) | 9 (4.5) |
| Diller | 1003 (93.1) | 101 (98.1) | 710 (91.8) | 192 (95.5) |
| Health status | ||||
| BMI, mean (SD) | 24.7 (5.1) | 24.4 (5.1) | 24.7 (5.0) | 25.0 (5.5) |
| General health, self-report | ||||
| Excellent | 413 (38.3) | 46 (44.7) | 295 (38.2) | 72 (35.8) |
| Good | 536 (49.8) | 51 (49.5) | 384 (49.7) | 101 (50.2) |
| Fair/poor | 128 (11.9) | 6 (5.8) | 94 (12.2) | 28 (13.9) |
| Postmenopausal | 733 (69.4) | 36 (35.6) | 544 (71.9) | 153 (77.3) |
| Personal history of any cancer | 757 (70.3) | 17 (16.5) | 609 (78.8) | 131 (65.2) |
| Personal history of breast cancer | 660 (61.3) | 10 (9.7) | 546 (70.6) | 104 (51.7) |
| Family cancer history | ||||
| First-degree relative with breast cancer | 433 (40.4) | 53 (51.5) | 276 (35.8) | 104 (52.3) |
| First-degree relative with ovarian cancer | 182 (17.0) | 35 (34.0) | 90 (11.7) | 57 (28.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Diller, Helen Diller Family Comprehensive Cancer Center; SFGH, San Francisco General Hospital.
Of these 1077 respondents, 201 received positive test results for BRCA1 or BRCA2 mutations (18.7%), 103 received a true-negative result (9.6%), 59 received a variant of undetermined significance (5.5%), and 714 received an uninformative negative result (66.3%). Overall, 773 women received uninformative results (71.8%). None of the women in our study who received uninformative negative test results had developed ovarian cancer during the follow-up period. Most of the study population was white (83.7%) and 38.7% was Ashkenazi Jewish (Table 2).
Predictors of RRSO
At the time of survey completion, 70.3% of respondents (757 of 1077) had at least 1 ovary. Two hundred six (19.1%) reported RRSO since BRCA testing, and the remaining 114 (10.6%) had their ovaries removed for other reasons (78 for ovarian cancer treatment and 36 for reasons other than risk reduction or ovarian cancer treatment). Seventy-two percent of women who reported removal of their ovaries for reasons other than risk reduction had undergone surgery before BRCA testing. Ovarian cancer was detected incidentally in 7 women during RRSO.
Among BRCA carriers, 69.6% reported undergoing RRSO after BRCA testing. An additional 12.3% of participants with uninformative and 2.0% with true-negative results reported RRSO. In multivariate analysis, women were 28.1 times more likely to undergo RRSO if they were BRCA carriers compared with women whose BRCA results were uninformative (Table 3); women who received true-negative results were significantly less likely to undergo RRSO. Additional multivariate predictors of RRSO included age 40 to 49 years at the time of RRSO, more than $500 000 in income-producing assets, 2 or more children, a history of breast cancer, and a first-degree relative with ovarian cancer.
Table 3. Characteristics of Women Who Underwent RRSO After BRCA Testing.
| No. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | No RRSO (n = 757) | RRSO (n = 206) | Adjusted OR (95% CI) | P Value |
| BRCA result | ||||
| Uninformative | 605 (87.7) | 85 (12.3) | 1 [Reference] | |
| Positive | 52 (30.4) | 119 (69.6) | 28.1 (16.2-48.6) | <.001 |
| True-negative | 100 (98.0) | 2 (2.0) | 0.1 (0.0-0.6) | .01 |
| Age at RRSO or survey, ya | ||||
| <40 | 106 (79.1) | 28 (20.9) | 1 [Reference] | |
| 40-49 | 216 (71.3) | 87 (28.7) | 2.6 (1.2-5.6) | .01 |
| 50-59 | 237 (78.7) | 64 (21.3) | 2.1 (1.0-4.6) | .06 |
| ≥60 | 198 (89.6) | 23 (10.4) | 0.8 (0.3-2.0) | .63 |
| Race | ||||
| Nonwhite | 120 (78.9) | 32 (21.1) | 1 [Reference] | |
| White | 636 (78.5) | 174 (21.5) | 1.2 (0.6-2.2) | .59 |
| Ashkenazi Jewishb | ||||
| No | 418 (78.3) | 116 (21.7) | 1 [Reference] | |
| Yes | 300 (78.7) | 81 (21.3) | 0.9 (0.6-1.4) | .63 |
| Income-producing assets, $ | <.001 | |||
| ≤100 000 | 148 (85.5) | 25 (14.5) | 1 [Reference] | |
| 100 001-500 000 | 255 (78.9) | 68 (21.1) | 1.8 (0.9-3.5) | .11 |
| 500 001-1 000 000 | 100 (74.1) | 35 (25.9) | 3.3 (1.5-7.4) | .004 |
| ≥1 000 001 | 254 (76.7) | 77 (23.3) | 3.5 (1.7-7.3) | <.001 |
| Testing sitec | ||||
| SFGH | 55 (88.7) | 7 (11.3) | ||
| Diller | 702 (77.9) | 199 (22.1) | ||
| BMI, mean (SD) | 24.4 (4.8) | 25.0 (5.6) | 1.0 (1.0-1.1) | .84 |
| No. of live births | ||||
| Mean (SD) | 1 (1) | 2 (1) | ||
| 0 | 226 (82.8) | 47 (17.2) | 1 [Reference] | |
| 1 | 163 (81.5) | 37 (18.5) | 1.3 (0.7-2.5) | .46 |
| ≥2 | 362 (74.9) | 121 (25.1) | 2.3 (1.3-3.9) | .003 |
| Personal history of breast cancer | 476 (77.4) | 139 (22.6) | 2.2 (1.3-3.7) | .004 |
| First-degree relative with breast cancer | 303 (75.4) | 99 (24.6) | 1.12 (0.8-1.8) | .48 |
| First-degree relative with ovarian cancer | 102 (61.1) | 65 (38.9) | 4.2 (2.4-7.4) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Diller, Helen Diller Family Comprehensive Cancer Center; OR, odds ratio; RRSO, risk-reducing salpingo-oophorectomy; SFGH, San Francisco General Hospital.
Age at RRSO for respondents reporting RRSO. For respondents who did not report RRSO, age at survey.
Forty-seven participants responded that they did not know whether they had Ashkenazi Jewish ancestry.
Testing site was not included in the final multivariate model because of the low absolute number of RRSOs (n = 7) in the SFGH population per the statistical analysis plan described in the “Methods” section.
Predictors of Ovarian Cancer Screening
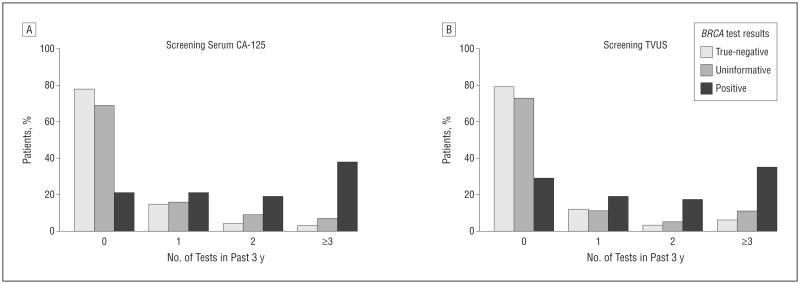
Among women with at least 1 ovary, 39.6% reported ever having received ovarian cancer screening by TVUS and 36.1% with serum CA-125. Of all the women surveyed, 39.6% underwent at least 1 screening test. Of eligible BRCA carriers, 26.3% underwent screening TVUS and 26.3% reported receiving serum CA-125 testing 3 or more times in the 3 years before the survey (Figure). Among participants with uninformative BRCA results, 33.8% and 37.3% ever received serum CA-125 or TVUS, respectively, since BRCA testing, while 10.4% and 6.5% received serum CA-125 or TVUS, respectively, 3 or more times in the 3 years prior to survey.
Figure.
Rate of ovarian cancer screening tests in the 3 years before the survey by BRCA results. A, Screening serum cancer antigen 125 (CA-125). B, Screening transvaginal ultrasonography (TVUS).
Predictors of TVUS and serum CA-125 screening were similar to predictors of undergoing RRSO, with the exception of a history of breast cancer, which increased the likelihood of RRSO but decreased the likelihood of screening interventions (Table 4 and Table 5). Women who received screening TVUS and screening serum CA-125 testing were more likely to be BRCA carriers or to have a first-degree relative with ovarian cancer and less likely to have received a true-negative result. We observed a trend toward higher screening rates among women aged 40 to 49 years and those who were tested at Diller, which did not achieve statistical significance for TVUS but was significant for serum CA-125. In contrast to RRSO, higher parity did not predict screening practices.
Table 4. Characteristics of Women Who Did Not Undergo RRSO and Received TVUS for Ovarian Cancer Screening in the Past Yeara.
| No. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | No TVUS (n = 596) | TVUS (n = 156) | Adjusted OR (95% CI) | P Value |
| BRCA result | ||||
| Uninformative | 491 (81.7) | 110 (18.3) | 1 [Reference] | |
| Positive | 15 (29.4) | 36 (70.6) | 9.5 (4.3-21.0) | <.001 |
| True-negative | 90 (90.0) | 10 (10.0) | 0.2 (0.1-0.5) | <.001 |
| Age at survey, y | ||||
| <40 | 70 (67.3) | 34 (32.7) | 1 [Reference] | |
| 40-49 | 164 (76.3) | 51 (23.7) | 1.5 (0.7-2.9) | .27 |
| 50-59 | 197 (83.5) | 39 (16.5) | 0.9 (0.4-1.8) | .75 |
| ≥60 | 165 (83.8) | 32 (16.2) | 1.1 (0.5-2.3) | .85 |
| Race | ||||
| Nonwhite | 93 (78.2) | 26 (21.8) | 1 [Reference] | |
| White | 503 (79.5) | 130 (20.5) | 0.9 (0.5-1.6) | .60 |
| Ashkenazi Jewishb | ||||
| No | 333 (80.0) | 83 (20.0) | 1 [Reference] | |
| Yes | 233 (78.2) | 65 (21.8) | 0.9 (0.6-1.5) | .79 |
| Income-producing assets, $ | ||||
| ≤100 000 | 114 (78.1) | 32 (21.9) | 1 [Reference] | |
| 100 001-500 000 | 191 (74.9) | 64 (25.1) | 0.9 (0.5-1.7) | .73 |
| 500 001-1 000 000 | 86 (86.0) | 14 (14.0) | 0.5 (0.2-1.1) | .09 |
| ≥1 000 001 | 205 (82.1) | 46 (18.3) | 1.1 (0.6-1.9) | .85 |
| Testing site | ||||
| SFGH | 48 (87.3) | 7 (12.7) | 1 [Reference] | |
| Diller | 548 (78.6) | 149 (21.4) | 2.3 (0.8-6.6) | .12 |
| BMI, mean (SD) | 24.5 (5.7) | 24.1 (5.1) | 1.0 (0.9-1.0) | .33 |
| No. of live births | ||||
| 0 | 167 (74.6) | 57 (25.4) | 1 [Reference] | |
| 1 | 130 (79.8) | 3 (20.2) | 1.2 (0.7-2.0) | .60 |
| ≥2 | 296 (82.0) | 65 (18.0) | 1.0 (0.6-1.6) | .91 |
| Personal history of breast cancer | 394 (83.5) | 78 (16.5) | 0.5 (0.3-0.9) | .01 |
| First-degree relative with breast cancer | 242 (80.1) | 60 (19.9) | 0.8 (0.5-1.1) | .19 |
| First-degree relative with ovarian cancer | 62 (60.8) | 40 (39.2) | 3.1 (1.8-5.6) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Diller, Helen Diller Family Comprehensive Cancer Center; OR, odds ratio; RRSO, risk-reducing salpingo-oophorectomy; SFGH, San Francisco General Hospital; TVUS, transvaginal ultrasonography.
Five participants did not respond to the survey question about TVUS.
Thirty-eight participants responded that they did not know whether they had Ashkenazi Jewish ancestry.
Table 5. Characteristics of Women Who Did Not Undergo RRSO and Received Serum CA-125 Testing for Ovarian Cancer Screening in the Past Yeara.
| No. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | No CA-125 (n = 592) | CA-125 (n = 156) | Adjusted OR (95% CI) | P Value |
| BRCA result | ||||
| Uninformative | 485 (81.4) | 111 (18.6) | 1 [Reference] | |
| Positive | 17 (32.7) | 35 (67.3) | 13.0 (5.5-29.0) | <.001 |
| True-negative | 90 (90.0) | 10 (10.0) | 0.3 (0.1-0.7) | .002 |
| Age at survey, y | ||||
| <40 | 76 (73.1) | 28 (26.9) | 1 [Reference] | |
| 40-49 | 163 (76.2) | 51 (23.8) | 2.4 (1.1-5.1) | .02 |
| 50-59 | 191 (82.0) | 42 (18.0) | 1.7 (0.8-3.8) | .16 |
| ≥60 | 162 (82.2) | 35 (17.8) | 2.0 (0.9-4.6) | .10 |
| Race | ||||
| Nonwhite | 88 (74.6) | 30 (25.4) | 1 [Reference] | |
| White | 504 (80.0) | 126 (20.0) | 0.7 (0.4-1.2) | .17 |
| Ashkenazi Jewishb | ||||
| No | 323 (77.8) | 92 (22.2) | 1 [Reference] | |
| Yes | 237 (80.1) | 59 (19.9) | 0.7 (0.5-1.1) | .16 |
| Income-producing assets, $ | ||||
| ≤100 000 | 113 (77.9) | 32 (22.1) | 1 [Reference] | |
| 100 001-500 000 | 189 (75.0) | 63 (25.0) | 1.1 (0.6-2.0) | .67 |
| 500 001-1 000 000 | 82 (82.0) | 18 (18.0) | 0.8 (0.4-1.6) | .47 |
| ≥1 000 001 | 208 (82.9) | 43 (17.1) | 0.8 (0.4-1.5) | .51 |
| Testing site | ||||
| SFGH | 48 (87.3) | 7 (12.7) | 1 [Reference] | |
| Diller | 544 (78.5) | 149 (21.5) | 4.3 (1.4-14.0) | .01 |
| BMI, mean (SD) | 24.4 (4.6) | 24.5 (5.3) | 1.0 (1.0-1.1) | .94 |
| No. of live births | ||||
| 0 | 169 (76.1) | 53 (23.9) | 1 [Reference] | |
| 1 | 122 (74.81) | 41 (25.2) | 1.8 (1.0-3.1) | .045 |
| ≥2 | 298 (83.0) | 61 (17.0) | 0.9 (0.5-1.4) | .57 |
| Personal history of breast cancer | 383 (82.0) | 84 (18.0) | 0.5 (0.3-0.8) | .009 |
| First-degree relative with breast cancer | 253 (84.3) | 47 (15.7) | 0.4 (0.2-0.6) | <.001 |
| First-degree relative with ovarian cancer | 64 (62.7) | 38 (37.3) | 2.3 (1.3-4.2) | .004 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CA-125, cancer antigen 125; Diller, Helen Diller Family Comprehensive Cancer Center; OR, odds ratio; RRSO, risk-reducing salpingo-oophorectomy; SFGH, San Francisco General Hospital.
Nine participants did not answer the survey question about CA-125 screening.
Seventeen participants reported not that they did not know whether they had Ashkenazi Jewish ancestry.
Comments
Women who may be at risk for hereditary ovarian cancer face difficult decisions after BRCA testing regardless of the test result. For BRCA carriers, the invasive and potentially life-altering nature of RRSO, as well as the ambiguity in screening recommendations should they forego or delay RRSO, contribute to these difficulties. For most women who receive uninformative results of BRCA testing, the lack of clear guidelines and the imprecise ability to predict the individual risk of ovarian cancer may foster uncertainty in patients and physicians. This study sheds light on current practices in ovarian cancer risk-reduction and screening interventions in both of these populations.
In this study, the women at highest risk—BRCA carriers—were the most likely to receive aggressive interventions, with a 69.6% use of RRSO and a 28-fold higher odds of receiving RRSO compared with women with uninformative BRCA results. The proportion of BRCA carriers in this study who underwent RRSO is at the higher end relative to rates reported in earlier studies6-10,28-30 of RRSO among BRCA carriers. One potential reason for the higher rate of RRSO in this study is its longer follow-up period of 3.7 years compared with 1 to 2 years in earlier studies.6,7,9 Another potential difference from previous studies relates to the fact that the population in the present study was surveyed during a period of rapidly accumulating evidence for the benefit of RRSO in BRCA carriers. In 2009, 1 year after our survey was administered, the American College of Obstetricians and Gynecologists31 published its guidelines recommending RRSO in BRCA carriers at age 40 years or when childbearing is complete. Our results suggest that BRCA carriers in this study are generally following these guidelines for RRSO.
In contrast, women in this study with true-negative BRCA results had significantly lower odds of receiving RRSO and ovarian cancer screening compared with women with uninformative BRCA results. Earlier studies32 indicated that women with true-negative BRCA results face a lifetime ovarian cancer risk of approximately 1% to 2%, which is similar to that of the general population. With this low lifetime risk, as well as the demonstrated harms of false-positive screening tests, there is insufficient evidence to recommend ovarian cancer screening for women with true-negative BRCA results.7,19-23
More than 70% of BRCA-tested women in this study—and in the United States—receive uninformative results.33 In this understudied but important population, a substantial proportion underwent RRSO (12.3%) and reported receiving ovarian cancer screening (30.2%) at least once in the preceding 3 years. Long-term ovarian cancer risk in women with uninformative BRCA results has not been carefully defined. Because this population likely represents a heterogeneous group, however, it is possible that certain subgroups, such as women with strong family histories of ovarian cancer, may be at higher-than-average risk. In the face of such uncertainty, patients and physicians may opt to obtain screening tests despite the absence of evidence-based guidelines and the questionable efficacy of these tests.31
For ovarian cancer, which is rare in the general population, most women do not benefit from screening, even those at highest risk.17,21,22 The most aggressive intervention to reduce risk, RRSO, has been carefully studied in BRCA carriers, and strong evidence supports its usefulness in reducing the incidence of ovarian cancer.4-6,19,34 Testing for BRCA in high-risk families can discriminate ovarian cancer risk and help women with positive and true-negative results determine whether further interventions are appropriate. For most women with uninformative BRCA results, RRSO and ovarian cancer screening may not be appropriate, barring strong family histories of ovarian cancer. The development of ovarian cancer would be expected to be a rare event during a median follow-up of 3.7 years, and none of the women who received uninformative negative test results subsequently received a diagnosis of ovarian cancer.
Several limitations of this study should be noted. As in all survey-based studies, available data were largely limited to self-reported survey responses, thereby introducing recall bias. Although the longer time since testing is a strength of our study, the lag between BRCA testing and survey completion could have allowed predictor variables to occur after the outcome of interest. There is the potential for volunteer bias in survey-based studies; however, there was no significant difference between responders and nonresponders in our analysis. Finally, respondents could have misinterpreted the survey questions, with subsequent inaccuracies in the data. Unmeasured disparities in health literacy may have augmented this effect, and, in particular, patients may have received the studied interventions for reasons other than risk reduction or screening. Additional large-scale, multi-institutional studies are needed to confirm the generalizability of our findings.
Our inability to assess the contribution of patient preferences and provider recommendations to the high prevalence of self-reported ovarian cancer screening is particularly important to this study. Practice setting or practitioner background may be an unmeasured predictor of behavior in this study. A recent vignette-based physician survey35 found that 6% routinely offer ovarian cancer screening to patients at low risk; this increased to 24% in women at medium risk, suggesting that physicians are partially driving increased screening rates. Physicians were also more likely to order ovarian cancer screening tests if requested by patients, regardless of their ovarian cancer risk. Future studies would benefit from surveying patient attitudes toward ovarian cancer screening and including provider characteristics in their analyses.
Strengths of this study include the large and diverse population, the excellent survey response rate, and the length of time since BRCA testing. To our knowledge, these data characterize ovarian cancer risk-reducing and screening interventions in one of the largest cohorts of high-risk women to date. In comparison with previous studies, the population included more racial and socioeconomic diversity.26 Although prior studies have typically examined either populations at average risk or populations of only BRCA carriers, the present study stratified participants according to BRCA test result and analyzed risk-reduction and screening interventions among all groups. In light of increasing data suggesting that screening, even in higher-risk women, may not improve outcomes, an updated assessment of screening prevalence may be beneficial as revised guidelines are considered.19
In summary, this study characterizes risk-reducing and screening interventions after BRCA test results in a large and diverse cohort of potentially high-risk women. We identified BRCA results to be the strongest predictor of RRSO and ovarian cancer screening in this study population. We also identified associations between breast cancer history and family history of ovarian cancer with RRSO and ovarian cancer screening. When ovarian cancer screening rates in the preceding 3 years were compared on the basis of BRCA results, we found that approximately 69.6% of BRCA carriers, 30.2% of women with uninformative BRCA results, and 9.6% of women with true-negative BRCA results reported having undergone screening. The RRSO and ovarian screening were reported in a sizable percentage of non-BRCA carriers despite insufficient data to determine the effectiveness of these interventions in this population.
Acknowledgments
Funding/Support: This research was supported by the UCSF Clinical Translational Science Institute, grant P01 CA130818-02A1 from the Center for Translational and Policy Research in Personalized Medicine, the Avon Foundation, grant 2P50 CA058207 from the Bay Area Breast Specialized Program of Research Excellence, and the Doris Duke Charitable Foundation.
Funding for Less Is More: Staff support for topics research funded by grants from the California Health Care Foundation and the Parsemus Foundation.
Footnotes
Author Contributions: Study concept and design: Mannis and Beattie. Acquisition of data: Beattie. Analysis and interpretation of data: Mannis, Fehniger, Creasman, Jacoby, and Beattie. Drafting of the manuscript: Mannis, Fehniger, and Jacoby. Critical revision of the manuscript for important intellectual content: Mannis, Fehniger, Creasman, Jacoby, and Beattie. Statistical analysis: Mannis, Creasman, and Jacoby. Obtained funding: Beattie. Administrative, technical, and material support: Fehniger.
Conflict of Interest Disclosures: None reported.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King MC, Marks JH, Mandell JB New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 4.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346(21):1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MD, Kaufman E, Peshkin BN, et al. Bilateral prophylactic oophorectomy and ovarian cancer screening following BRCA1/BRCA2 mutation testing. J Clin Oncol. 2003;21(21):4034–4041. doi: 10.1200/JCO.2003.01.088. [DOI] [PubMed] [Google Scholar]
- 8.Uyei A, Peterson SK, Erlichman J, et al. Association between clinical characteristics and risk-reduction interventions in women who underwent BRCA1 and BRCA2 testing: a single-institution study. Cancer. 2006;107(12):2745–2751. doi: 10.1002/cncr.22352. [DOI] [PubMed] [Google Scholar]
- 9.Madalinska JB, van Beurden M, Bleiker EM, et al. Predictors of prophylactic bilateral salpingo-oophorectomy compared with gynecologic screening use in BRCA1/2 mutation carriers. J Clin Oncol. 2007;25(3):301–307. doi: 10.1200/JCO.2006.07.4922. [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, et al. Hereditary Breast Cancer Clinical Study Group. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2008;122(9):2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beattie MS, Crawford B, Lin F, Vittinghoff E, Ziegler J. Uptake, time course, and predictors of risk-reducing surgeries in BRCA carriers. Genet Test Mol Biomarkers. 2009;13(1):51–56. doi: 10.1089/gtmb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Cancer Society. [Accessed January 23, 2012];Can ovarian cancer be found early? Updated October 2010. http://www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-detection.
- 13.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee opinion No. 477: the role of the generalist obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol. 2011;117(3):742–746. doi: 10.1097/AOG.0b013e31821477db. [DOI] [PubMed] [Google Scholar]
- 14.Gladstone CQ. Screening for ovarian cancer. In: Canadian Task Force on the Periodic Health Examination, editor. Canadian Guide to Clinical Preventive Health Care. Ottawa, ON: Minister of Public Works and Government Services Canada; 1994. pp. 870–881. [Google Scholar]
- 15.National Comprehensive Cancer Network Practice Guidelines in Oncology. [Accessed January 23, 2012];Genetic familial high-risk assessment: breast and ovarian. Updated January 2011. https://subscriptions.nccn.org/gl_login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- 16.US Preventive Services Task Force. Screening for ovarian cancer: recommendation statement. Am Fam Physician. 2005;71(4):759–762. [PubMed] [Google Scholar]
- 17.Hermsen BB, Olivier RI, Verheijen RH, et al. No efficacy of annual gynaecological screening in BRCA1/2 mutation carriers; an observational follow-up study. Br J Cancer. 2007;96(9):1335–1342. doi: 10.1038/sj.bjc.6603725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Nagell JR, Jr, DePriest PD, Ueland FR, et al. Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened. Cancer. 2007;109(9):1887–1896. doi: 10.1002/cncr.22594. [DOI] [PubMed] [Google Scholar]
- 19.Buys SS, Partridge E, Black A, et al. PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening randomized controlled trial. JAMA. 2011;305(22):2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 20.van Nagell JR, Jr, Miller RW, DeSimone CP, et al. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet Gynecol. 2011;118(6):1212–1221. doi: 10.1097/AOG.0b013e318238d030. [DOI] [PubMed] [Google Scholar]
- 21.Woodward ER, Sleightholme HV, Considine AM, Williamson S, McHugo JM, Cruger DG. Annual surveillance by CA125 and transvaginal ultrasound for ovarian cancer in both high-risk and population risk women is ineffective. BJOG. 2007;114(12):1500–1509. doi: 10.1111/j.1471-0528.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaarenstroom KN, van der Hiel B, Tollenaar RA, et al. Efficacy of screening women at high risk of hereditary ovarian cancer: results of an 11-year cohort study. Int J Gynecol Cancer. 2006;16(suppl 1):54–59. doi: 10.1111/j.1525-1438.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 23.Laframboise S, Nedelcu R, Murphy J, Cole DE, Rosen B. Use of CA-125 and ultrasound in high-risk women. Int J Gynecol Cancer. 2002;12(1):86–91. doi: 10.1046/j.1525-1438.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 24.van der Velde NM, Mourits MJ, Arts HJ, et al. Time to stop ovarian cancer screening in BRCA1/2 mutation carriers? Int J Cancer. 2009;124(4):919–923. doi: 10.1002/ijc.24038. [DOI] [PubMed] [Google Scholar]
- 25.Parmigiani G, Berry DA, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62(1):145–158. doi: 10.1086/301670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee R, Beattie M, Crawford B, et al. Recruitment, genetic counseling, and BRCA testing for underserved women at a public hospital. Genet Test. 2005;9(4):306–312. doi: 10.1089/gte.2005.9.306. [DOI] [PubMed] [Google Scholar]
- 27.Claritas. [Accessed January 23, 2012];The Nielsen Co: income producing assets and net worth profiles: assessing wealth by segment targeting. Updated 2008. http://www.clusterstaging.claritas.com/collateral/segmentation/targeting-by-segment_f3026.pdf.
- 28.Manchanda R, Burnell M, Abdelraheim A, et al. Factors influencing uptake and timing of risk reducing salpingo-oophorectomy in women at risk of familial ovarian cancer: a competing risk time to event analysis. BJOG. 2012;119(5):527–536. doi: 10.1111/j.1471-0528.2011.03257.x. [DOI] [PubMed] [Google Scholar]
- 29.Sidon L, Ingham S, Clancy T, et al. Uptake of risk-reducing salpingo-oophorectomy in women carrying a BRCA1 or BRCA2 mutation: evidence for lower uptake in women affected by breast cancer and older women. Br J Cancer. 2012;106(4):775–779. doi: 10.1038/bjc.2011.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz MD, Isaacs C, Graves KD, et al. Long-term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer. 2012;118(2):510–517. doi: 10.1002/cncr.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu K, Kauff N, Powell CB, et al. American College of Obstetricians and Gynecologists; ACOG Committee on Practice Bulletins–Gynecology; ACOG Committee on Genetics; Society of Gynecologic Oncologists. ACOG Practice Bulletin No. 103: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2009;113(4):957–966. doi: 10.1097/AOG.0b013e3181a106d4. [DOI] [PubMed] [Google Scholar]
- 32.Kauff ND, Mitra N, Robson ME, et al. Risk of ovarian cancer in BRCA1 and BRCA2 mutation-negative hereditary breast cancer families. J Natl Cancer Inst. 2005;97(18):1382–1384. doi: 10.1093/jnci/dji281. [DOI] [PubMed] [Google Scholar]
- 33.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20(6):1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 34.Press DJ, Sullivan-Halley J, Ursin G, et al. Breast cancer risk and ovariectomy, hysterectomy, and tubal sterilization in the Women's Contraceptive and Reproductive Experiences Study. Am J Epidemiol. 2011;173(1):38–47. doi: 10.1093/aje/kwq339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin LM, Trivers KF, Matthews B, et al. Vignette-based study of ovarian cancer screening: do US physicians report adhering to evidence-based recommendations? Ann Intern Med. 2012;156(3):182–194. doi: 10.7326/0003-4819-156-3-201202070-00006. [DOI] [PubMed] [Google Scholar]