Cholera has been for many centuries a permanent feature of life in the slums and poverty-stricken villages in India, particularly in the eastern regions of the country. In this region outbreaks have occurred frequently since the early 1800s1. The first six cholera pandemics originated from this geographical region while the seventh and ongoing pandemic originated in 1961 from Sulawasi in Indonesia. Most recent evidence indicates that within the seventh cholera pandemic two of the three waves of the El Tor biotype of Vibrio cholerae originated from the Gangetic delta off the Bay of Bengal2.
Recent estimates have shown 2.9 million cases and 95,000 deaths occurring annually in cholera endemic countries worldwide3. Of this, India accounts for 675,188 cases and 20,256 deaths3. In addition, an issue of concern is that 65-86 per cent of the isolates have been found to be resistant to the commonly used antimicrobials and their prevalence is concentrated in the lowest quintile of wealth4.
Although access to clean water for drinking and hand washing and good sanitation system form the pillars of cholera prevention and control, availability of a reasonably priced, safe and effective vaccine provides an additional intervention for quick deployment and protection against the disease. One of the WHO prequalified oral cholera vaccine (OCV) is manufactured in India and has been found safe and effective in endemic as well as epidemic situations in countries around the globe5. Although this vaccine is in use in the private health care system in India, it has not been considered for use in the national immunization programme of the country. Here we analyze the possible reasons why OCVs have not been used in India in the national immunization programme, through which these vaccines could be made available to people who need it the most.
Cholera: the disease
V. cholera is transmitted through contaminated water and food and is associated with poverty, poor hygiene, and inadequate sanitation. The disease typically begins with an acute attack of watery diarrhoea and copious vomiting, rapidly followed by dehydration and in the absence of treatment, renal failure and death can occur within a matter of 3 to 4 h. About 80 per cent of cholera episodes are of mild-to-moderate severity. Cholera usually responds to antibiotics and prompt administration of oral rehydration salts (ORS) to replace lost fluids. In the past, before the advent of fluid replacement therapy, up to 50 per cent of infected people died from the disease6. Despite the case fatality reducing to less than 5 per cent, on a global average6, the incidence of cholera remains the same in the past decades7. Since the average duration of an episode of paediatric diarrhoea is 3.1 days, and approximately a quarter of those are of mild presentation, caregivers are reluctant to seek any form of treatment. Although ORS is recommended for all episodes at the population level, the rate of its usage is only 75 per cent at the maximum globally8. In India, the utilization of ORS hovers around 23 to 42 per cent9. In severe cases, intravenous (iv) infusion of fluids is mandatory, for which hospitalization is required is also often out of reach of the affected population.
Water, sanitation and hygiene (WaSH) in prevention of cholera
Provision of clean drinking water, improved sanitation and knowledge about hygiene form the mainstay of prevention of any disease transmitted by the faeco-oral route. These interventions have been used as long-term measures for cholera prevention and control. However, these are resource-intensive as well as time-consuming to implement. Despite heavy investments in these interventions globally, a significant proportion of population still remains unreached. Although global target for Millennium Development Goals (MDG) for drinking-water has been met in 2010, only 147 countries have met the goal as of 201510. Disparities exist between those living in rural and urban areas and pose a challenge to achieving equity in health. Moreover, available information about drinking water safety is incomplete, as systematic testing of the quality of water, both microbial and chemical, at a national level in these countries is prohibitively expensive and logistically complicated10.
Unplanned migration from rural to urban areas in search of employment, particularly in the unorganized sector, is leading to a changing pattern of demographics. This causes overcrowding in the urban slums, where density of population is ever exceeding the available resources. Susceptibility to diarrhoeal diseases like cholera is aggravated by scarcity of potable water and lack of proper sanitation in such urban slums.
Another factor that complicates issues in this case is the practice of open defaecation. Approximately 15 per cent of world's population i.e. 1.1 billion people practice open-defaecation, often contaminating the sources of drinking water. India has almost 50 per cent of its rural population defaecating in the open, which amounts to nearly 60 per cent of the global total10.
Cholera vaccines
Vaccines for cholera have a short-term effect (3-5 years), but are easily-deployable and evoke an immediate response, thus making them remarkable public health tools in both endemic as well as epidemic situations11,12. The realization that a vaccine could prevent the disease, originated in the days of Robert Koch, who is credited with the discovery of cholera, the “comma” bacillus. Since then, a number of injectable whole cell based vaccines have been developed. However, these parenteral cholera vaccines were discontinued by WHO in the early 1970s, because of the realization that the injectable cholera vaccine was more painful than protective. The parenteral vaccine conferred 30-50 per cent protection for 3-5 months after intramuscular (im) or subcutaneous (sc) administration and caused local erythema, pain, fever, headache and malaise13.
Currently, two oral cholera vaccines are WHO prequalified and are available. Of these, Dukoral® made from the inactivated whole-cell V. cholera with a recombinant fragment (B subunit) of the cholera toxin is manufactured by SBL Vaccin AB (owned by Crucell, Sweden AB). The latter component conferred additional protection against enterotoxigenic Escherichia coli (ETEC) apart from cholera14,15. Field trials in Bangladesh16, Mozambique17, and Peru18 found the vaccine to be safe and effective. But the vaccine has some shortcomings. First, it requires two doses given one week apart and requires to be taken with a buffer solution; the two factors that complicate its use, particularly in epidemic situation. Second, its protective capability takes about three weeks to develop after administration of the first dose. Protection is highest during the first six months after vaccination but lasts for up to three years16. Third, it is effective only against the V. cholera O1 serogroup, which until 1992, was the only serogroup causing cholera. But in 1992 a second serogroup, O13919,20,21 was identified as the cause of epidemics in Bangladesh and India, and has since been implicated in a growing number of outbreaks in Asia22,23.
The second vaccine, reformulated by the International Vaccine Institute (IVI), Korea, is now manufactured by Shantha Biotech (a Sanofi Company), based in Hyderabad and is marketed as Shanchol™. This vaccine is bivalent (BivWC) as it incorporates newly emerged O139 serogroup apart from O1. Unlike its predecessor, this vaccine is cheap ( 120 per dose, as opposed to
120 per dose, as opposed to  320 for Dukoral®) and effective, with a vaccine efficacy of 65 per cent lasting for up to five years post-vaccination and possibly longer24. Unlike Dukoral®, ShancholTM does not require buffer thereby making it more acceptable for infants and children. Following its licensure in India, feasibility and costs of a local vaccination campaign using government health staff, resources, and logistics for Shanchol™ has been demonstrated in Odisha25. This demonstration project showed reduction in cholera cases by 69 per cent in the area over next two years26. The delivery of the vaccine is feasible in a public health system and there is little difference between overall protective effectiveness of the vaccine alone, as with other interventions like washing hands and using chlorinated water27.
320 for Dukoral®) and effective, with a vaccine efficacy of 65 per cent lasting for up to five years post-vaccination and possibly longer24. Unlike Dukoral®, ShancholTM does not require buffer thereby making it more acceptable for infants and children. Following its licensure in India, feasibility and costs of a local vaccination campaign using government health staff, resources, and logistics for Shanchol™ has been demonstrated in Odisha25. This demonstration project showed reduction in cholera cases by 69 per cent in the area over next two years26. The delivery of the vaccine is feasible in a public health system and there is little difference between overall protective effectiveness of the vaccine alone, as with other interventions like washing hands and using chlorinated water27.
Epidemics of cholera occur with regularity in India, among the marginalized people especially in remote areas where the social, epidemiological and ecological conditions continue to favour the occurrence of cholera. Therefore, it is enigmatic that in a country like India where the disease occurs in sporadic, endemic and epidemic proportions and where a safe efficacious oral cholera vaccine is available, vaccination against cholera is not considered an option in the country's public health programme. Obviously, there is a gap in our understanding as to why an available vaccine is not being used. We have critically analyzed the possible reasons for this enigma for deployment and uptake at different levels.
The possible reasons are as follows:
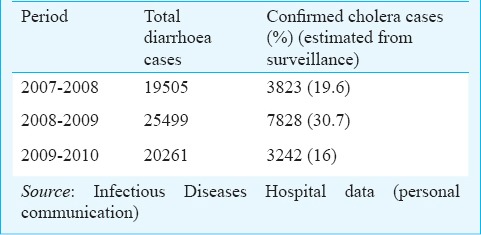
1 (i). Underreporting: Cholera continues to be grossly underreported in India. The extent of underreporting can be illustrated by comparing the number of cholera cases reported by India to the WHO (Table I) and the number of cholera cases admitted to the Infectious Diseases (ID) Hospital in Kolkata (Table II). Of note, is the fact that these hospitalization figures are for a single hospital in the country, which is more than the figures reported to WHO for the entire country for the entire year. Thus much lower numbers of cholera cases are recorded in the WHO database than that actually occur in the country. The major source of data for the Government is the Integrated Disease Surveillance Project (IDSP), which is a government of India initiative28. This originates at the State level and is compiled at the national level. However, there are several reasons for which cholera is underreported in this surveillance. The major cause for underreporting by clinicians is the non-availability of a rapid diagnostic test (RDT) for cholera at the point of care, as a result most of the cholera cases get reported as “acute diarrhoeal disease” (by a factor of 6)29. In addition, inconsistencies in case-definition also add to the dilemma. The underreported data are being used by the decision makers in the country, leading to the perception that the cholera does not exist in the country. Efforts must be made to obtain a realistic idea of the burden of cholera in India from available data and from robust estimations.
Table I.
Cholera cases and deaths reported by India to WHO, 2007-2010
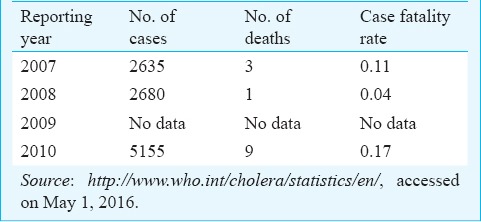
Table II.
Actual and estimated cholera cases at the Infectious Diseases Hospital, Kolkata, 2007-2010
1 (ii). The differing clinical characteristics of cholera: From the clinical spectrum of chlolera30, it is evident that the classical rice water stool is present only in cases of severe infection (cholera gravis), while in most mild infections, it is the loose or watery stool that is indistinguishable from other causes of diarrhoea. In asymptomatic infections, the stool is essentially normal. If we look at the latest figures of cholera and acute diarrhoeal disease cases from the National Health Profile of India 201231, it is quite evident that the number of cholera cases is miniscule when compared to the number of cases due to acute diarrhoeal diseases. Ironically in States like Odisha, where cholera is known to be endemic, there is no representation of cholera cases for 2012, although there are 740,000 cases of acute diarrhoeal disease with 235 deaths31.
1 (iii). Misperception of cholera among physicians and lay persons: The consistent underreporting of cholera in India has a snowballing effect, resulting in misperception of cholera among clinicians in the country. This could be because most of the clinicians, especially the ones trained / practicing in non-endemic areas or who have an affluent clientage, do not recognize a case of cholera. A concerted effort from different stakeholders is needed to inform the practicing physicians of the disease, its sub-geographic persistence and fast spread to other areas of the country. Most of the acute diarrhoeal cases go to primary health centers (PHCs) for treatment, rather than being treated by private medical practitioners, especially in rural areas. In urban settings in endemic areas, it is the hospitals attached to medical colleges that cater to the acute diarrhoeal cases. PHCs in endemic areas do not have accurate and sensitive diagnostic tests; and where these are available, personnel are not trained to use them32.
1 (iv). Political and socio-economic considerations: Outbreaks of cholera are rightly equated to breach in sanitation and lack of safe drinking water, which reflects poorly on the State health machinery. Consequently there is, at times, a tendency to push the episode under the carpet. Apart from the technical reasons, the gross underestimation could be attributed to other reasons, including the stigma attached to cholera in the society, for the fear of economic sanctions that follow. This situation continues despite the fact that trade embargoes have been lifted as far back as 200233. Such an important decision about the lifting of embargoes needs to be properly disseminated to a wider audience, ranging from key decision makers, on the one hand, to the layman on the other. Moreover, in India, V. cholerae is still considered a pathogen only for outbreak investigations, rather than part of national disease surveillance network.
1 (v). Lack of awareness in the community: The quantum of demand of any intervention from its potential users forms a very important criterion for decision making for its introduction in a public programme. A deeper inquiry into the handicaps at the patient level is also required to understand why a strong demand for OCV has not been generated so far. The ignorance about the disease and available preventive measures remains the major causes. The families that get affected in endemic areas are generally poor with a median monthly income of  4280 for a family of five members34,35. The onslaught of diarrhoeal diseases like cholera, coupled with poverty, forms a vicious cycle from which there is little hope of escape. Despite improvements in access to health care, inequities are related to socio-economic status, geography and gender and are compounded by high out-of-pocket expenditures, with more than three-quarters of the increasing financial burden of health care being met by households. Health care expenditures exacerbate poverty, with about 39 million additional people falling into poverty every year as a result of such expenditure36.
4280 for a family of five members34,35. The onslaught of diarrhoeal diseases like cholera, coupled with poverty, forms a vicious cycle from which there is little hope of escape. Despite improvements in access to health care, inequities are related to socio-economic status, geography and gender and are compounded by high out-of-pocket expenditures, with more than three-quarters of the increasing financial burden of health care being met by households. Health care expenditures exacerbate poverty, with about 39 million additional people falling into poverty every year as a result of such expenditure36.
There is a lack of awareness about availability of effective prevention measures like an OCV and its cost among the users. The new OCV is available at the cost of  120 per dose25, which amounts to 1.24 per cent of the income of the potential users as compared to 21 per cent for the cost of treatment37. However, those who are informed about the usefulness of a cholera vaccine are ready to receive the vaccine only if it is provided free38.
120 per dose25, which amounts to 1.24 per cent of the income of the potential users as compared to 21 per cent for the cost of treatment37. However, those who are informed about the usefulness of a cholera vaccine are ready to receive the vaccine only if it is provided free38.
2. Paradox at the vaccine manufacture and supply level: The continuous and adequate supply of the vaccine is a pre-requisite for it to be introduced in a publicly funded programme. The three steps that need to follow in tandem in order to encourage new manufacturers are (i) assessment of demand in India and around the globe, (ii) assessing the willingness of new vaccine manufacturers in developing countries to receive the technology and invest in manufacturing this vaccine, and (iii) have incentives in place to mitigate the risk to new manufacturers39.
The vaccine ShancholTM has been licensed in India since February 2009; and was WHO prequalified in September 201140, following its use in the Haiti outbreak41. GAVI, the Vaccine Alliance (previously called Global Alliance for Vaccines and Immunization) has pledged support for 20 million doses for the global stockpile over the next five years42. Lack of a firm demand fails to generate enough market-pull for companies to remain interested in the business. On the other hand, depending on only one producer for global supply of any vaccine is a significant risk for programmes, because of wavering business goals of companies. This situation is likely to improve with another OCV from Eubiologics in Korea (EuviChol) getting WHO prequalified43.
3. The missing champion for cholera in India: Several successful national public health programmes around the globe have had “Champions” behind them. Good examples include Gary Valenciano, a UNICEF National Ambassador for ORS in the Philippines and Paul Farmer for the use of OCV in Haiti. In India a “Champion” for cholera vaccine is still missing.
4. New advocacy and policy initiatives: There are a number of initiatives, where global strategies for cholera vaccination have been discussed, deliberated, and gaps and evidence identified. The Initiative against Diarrheal and Enteric diseases in Asia (IDEA) is one such initiative (http://www.idea-asia.info). This initiative, headquartered in Lyon, France, includes experts from eight cholera-endemic countries of Asia, who meet annually to take forward the agenda of cholera, both globally, as well as regionally. So far, four meetings have been convened in Chiang Mai, Thailand; Yogyakarta, Indonesia; Tagaytay City, the Philippines and New Delhi, India.
The Coalition for Cholera Prevention and Control (CCPC) is another new initiative that was established in 2011 through a 2-year conference grant from the Bill and Melinda Gates Foundation (BMGF) awarded to Task Force for Global Health (TFGH) and Harvard Medical School/Partners in Health (HMS/PIH) (http://choleracoalition.org). The CCPC is a coalition of partners focused on developing a comprehensive strategy for cholera prevention and control, including appropriate use of OCV in both endemic and epidemic settings. Several meetings have been held in the interim period since its inception and currently, the coalition members are working to gain endorsement of a strategy and mobilize resources for implementation of OCV.
The WHO Strategic Advisory Group of Experts (SAGE) on Oral Cholera vaccines was established in November 2015 (www.who.int/immunization/policy/sage/sage_wg_cholera_nov2015/en/). Their main objective is to analyse the results of Monitoring and Evaluation (M&E) activities implemented during OCV campaigns with particular focus on communities’ acceptability, safety of OCV, vaccine effectiveness in various settings, cost analysis, impact of cholera transmission in endemic and epidemic settings. Further, they are to review evidence and propose recommendations for vaccination strategies, for use of OCV in pregnant and lactating women as well as among travellers and propose potential revisions for endemic settings (“hotspots”) during humanitarian emergencies and during outbreaks.
Another initiative, known as the Stop Cholera or DOVE (Delivering Oral Vaccine Effectively) programme is spearheaded by Johns Hopkins Bloomberg School of Public Health (https://www.stopcholera.org). It includes a strong international team of experts. Its collaborators include the Bill and Melinda Gates Foundation, The African Cholera Surveillance Network (AfriCHOL), International Vaccine Institute, United Nations Children Fund, and the International Federation of Red Cross and Red Crescent Societies, to name a few. It has developed a number of important resources in the area of cholera prevention and control. This programme recommends that the OCV should be a part of comprehensive cholera control strategies.
In the absence of a national surveillance programme for cholera in India, data on burden and effectiveness of OCV in a national immunization programme are being modelled based on data from Bangladesh. Campaign and continuous vaccination targeting all individuals one year and older in 50 per cent in the population is projected to reduce cholera cases by close to 50 and 70 per cent, respectively44.
Way forward
It is quite evident that by looking at a decade long outbreak pattern, West Bengal has reported cholera outbreaks in all 10 years, followed by Maharashtra and Delhi (9 years), then Odisha (7 years)29. In recent years these outbreaks have spread to newer areas. These data give us a starting point for vaccination campaigns against cholera in India. Importantly, West Bengal seems to be a plausible place to begin vaccination efforts since evidence exists for at least two of three waves of global transmission in the seventh cholera pandemic originating essentially from this State in India1,2. This will require a concerted and sustained effort on the part of a small group of experts, dedicated specifically for cholera control in the country, to achieve the desired objective.
A cholera expert group has been constituted by the Department of Biotechnology, India with leading authorities in the field to address the issue of introduction of an OCV as an adjunct to other long-term methods such as WaSH in a holistic and well coordinated fashion, which will be time-bound and goal-oriented. Introduction of cholera vaccine piggybacking on the rotavirus vaccine might prove to be more effective from a logistical standpoint. Efforts are ongoing to take this initiative forward so that vaccination against cholera is used in at least one cholera endemic State in India, and serve as a model for other endemic States.
Footnotes
Conflicts of Interest: None.
References
- 1.Ramamurthy T, Sharma NC. Cholera outbreaks in India. Curr Top Microbiol Immunol. 2014;379:49–85. doi: 10.1007/82_2014_368. [DOI] [PubMed] [Google Scholar]
- 2.Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S, et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature. 2011;477:462–5. doi: 10.1038/nature10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9:e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sur D, Deen JL, Manna B, Niyogi SK, Deb AK, Kanungo S, et al. The burden of cholera in the slums of Kolkata, India: data from a prospective, community based study. Arch Dis Child. 2005;90:1175–81. doi: 10.1136/adc.2004.071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oral cholera vaccination facts. [accessed on April 20, 2016]. Available from: https://http://www.stopcholera.org/
- 6.Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–28. [PubMed] [Google Scholar]
- 7.Sarkar BL, Kanungo S, Nair GB. How endemic is cholera in India? Indian J Med Res. 2012;135:246–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson SE, Morris SS, Gilbert SS, Mosites E, Hackleman R, Weum KL, et al. Scaling up access to oral rehydration solution for diarrhea: Learning from historical experience in low- and high-performing countries. J Glob Health. 2013;3:010404. doi: 10.7189/jogh.03.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow J, Darley SR, Laxminarayan R. Resources for Future. Washington DC: 2007. Cost-effectiveness of disease interventions in India, Discussion series. [Google Scholar]
- 10.WHO/UNICEF Joint Monitoring Programme (JMP) for Water Supply and Sanitation 2015. [accessed on December 8, 2014]. Available from: http://www.wssinfo.org .
- 11.Luquero FJ, Grout L, Ciglenecki I, Sakoba K, Traore B, Heile M, et al. Use of Vibrio cholerae vaccine in an outbreak in Guinea. N Engl J Med. 2014;370:2111–20. doi: 10.1056/NEJMoa1312680. [DOI] [PubMed] [Google Scholar]
- 12.Luqueor FJ, Sack DA. Effectiveness of oral cholera vaccine in Haiti. Lancet Glob Health. 2015;3:e120–1. doi: 10.1016/S2214-109X(15)70015-X. [DOI] [PubMed] [Google Scholar]
- 13.Ryan ET, Calderwood SB. Cholera vaccines. Clin Infect Dis. 2000;31:561–5. doi: 10.1086/313951. [DOI] [PubMed] [Google Scholar]
- 14.Jelinek T, Kollaritsch H. Vaccination with Dukoral against travelers’ diarrhea (ETEC) and cholera. Expert Rev Vaccines. 2008;7:561–7. doi: 10.1586/14760584.7.5.561. [DOI] [PubMed] [Google Scholar]
- 15.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012;379:2466–76. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–3. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 17.Lucas ME, Deen JL, von Seidlein L, Wang XY, Ampuero J, Puri M, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005;352:757–67. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez JL, Hayashi KE, Kruger HF, Meza R, English CK, Vidal W, et al. Immunological response to Vibrio cholerae O1 infection and an oral cholera vaccine among Peruvians. Trans R Soc Trop Med Hyg. 1995;89:542–5. doi: 10.1016/0035-9203(95)90103-5. [DOI] [PubMed] [Google Scholar]
- 19.Garg S, Saha PK, Ramamurthy T, Deb BC, Nair GB, Shimada T, et al. Nationwide prevalence of the new epidemic strain of Vibrio cholerae O139 Bengal in India. J Infect. 1993;27:108–9. doi: 10.1016/0163-4453(93)94398-u. [DOI] [PubMed] [Google Scholar]
- 20.Faruque SM, Sack DA, Sack RB, Colwell RR, Takeda Y, Nair GB. Emergence and evolution of Vibrio cholerae O139. Proc Natl Acad Sci USA. 2003;100:1304–9. doi: 10.1073/pnas.0337468100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramamurthy T, Yamasaki S, Takeda Y, Nair GB. Vibrio cholerae O139 Bengal: odyssey of a fortuitous variant. Microbes Infect. 2003;5:329–44. doi: 10.1016/s1286-4579(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 22.Nusrin S, Khan GY, Bhuiyan NA, Ansaruzzaman M, Hossain MA, Safa A, et al. Diverse CTX phages among toxigenic Vibrio cholerae O1 and O139 strains isolated between 1994 and 2002 in an area where cholera is endemic in Bangladesh. J Clin Microbiol. 2004;42:5854–6. doi: 10.1128/JCM.42.12.5854-5856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz BS, Harris JB, Khan AI, Larcocque RC, Sack DA, Malek MA, et al. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg. 2006;74:1067–73. [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2013;13:1050–6. doi: 10.1016/S1473-3099(13)70273-1. [DOI] [PubMed] [Google Scholar]
- 25.Kar SK, Sah B, Patnaik B, Kim YH, Kerketta AS, Shin S, et al. Mass vaccination with a new, less expensive oral cholera vaccine using public health infrastructure in India: the Odisha model. PLoS Negl Trop Dis. 2014;8:e2629. doi: 10.1371/journal.pntd.0002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wierzba TF, Kar SK, Mogasale VV, Kerketta AS, You YA, Baral P, et al. Effectiveness of an oral cholera vaccine campaign to prevent clinically-significant cholera in Odisha State, India. Vaccine. 2015;33:2463–9. doi: 10.1016/j.vaccine.2015.03.073. [DOI] [PubMed] [Google Scholar]
- 27.Qadri F, Ali M, Chowdhury F, Khan AI, Saha A, Khan IA, et al. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet. 2015;386:1362–71. doi: 10.1016/S0140-6736(15)61140-0. [DOI] [PubMed] [Google Scholar]
- 28.Integrated Disease Surveillance Project. [accessed on April 20, 2016]. Available from: http://www.idsp.nic.in/
- 29.Kanungo S, Sah BK, Lopez AL, Sung JS, Paisley AM, Sur D, et al. Cholera in India: an analysis of reports, 1997-2006. Bull World Health Organ. 2010;88:185–91. doi: 10.2471/BLT.09.073460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson EJ, Harris JB, Morris JG, Jr, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009;7:693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Health Status Indicators. National Health Profile of India. 2012. [accessed on May 14, 2014]. Available from: http://www.cbhidghs.nic.in/writereaddata/mainlinkFile/Health Status Indicators-2012.pdf .
- 32.Kalluri P, Naheed A, Rahman S, Ansaruzzaman M, Faruque AS, Bird M, et al. Evaluation of three rapid diagnostic tests for cholera: does the skill level of the technician matter? Trop Med Int Health. 2006;11:49–55. doi: 10.1111/j.1365-3156.2005.01539.x. [DOI] [PubMed] [Google Scholar]
- 33.Aginam O. International law and communicable diseases. Bull World Health Organ. 2002;80:946–51. [PMC free article] [PubMed] [Google Scholar]
- 34.Sur D, Manna B, Deb AK, Deen JL, Danovaro-Holliday MC, von Seidlein L, et al. Factors associated with reported diarrhoea episodes and treatment-seeking in an urban slum of Kolkata, India. J Health Popul Nutr. 2004;22:130–8. [PubMed] [Google Scholar]
- 35.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut-a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–9. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balarajan Y, Selvaraj S, Subramanian SV. Health care and equity in India. Lancet. 2011;377:505–5. doi: 10.1016/S0140-6736(10)61894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulos C, Riewpaiboon A, Stewart JF, Clemens J, Guh S, Agtini M, et al. Costs of illness due to endemic cholera. Epidemiol Infect. 2012;140:500–9. doi: 10.1017/S0950268811000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wittington D, Sur D, Cook J, Chatterjee S, Maskery B. Rethinking cholera and typhoid vaccination policies for the poor: private demand in Kolkatta, India. World Dev. 2009;37:399–409. [Google Scholar]
- 39.Brogan D, Mossialos E. Applying the concepts of financial options to stimulate vaccine development. Nat Rev Drug discov. 2006;5:641–7. doi: 10.1038/nrd2035. [DOI] [PubMed] [Google Scholar]
- 40.Lopez AL, Gonzales ML, Aldaba JG, Nair GB. Killed oral cholera vaccines: history, development and implementation challenges. Ther Adv Vaccines. 2014;2:123–36. doi: 10.1177/2051013614537819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rouzier V, Severe K, Juste MA, Peck M, Perodin C, Severe P, et al. Cholera vaccination in urban Haiti. Am J Trop Med Hyg. 2013;89:671–81. doi: 10.4269/ajtmh.13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pape JW, Rouzier V. Embracing oral cholera vaccine-shifting response to cholera. N Engl J Med. 2014;370:2067–9. doi: 10.1056/NEJMp1402837. [DOI] [PubMed] [Google Scholar]
- 43.Cholera vaccine supply set to double, easing global shortage. [accessed on March 12, 2016]. Available from: http://www.who.int/cholera/vaccines/double/en/
- 44.Dimitrov DT, Troeger C, Halloran ME, Longini IM, Chao DL. Comparative effectiveness of different strategies of oral cholera vaccination in bangladesh: a modeling study. PLoS Negl Trop Dis. 2014;8:e3343. doi: 10.1371/journal.pntd.0003343. [DOI] [PMC free article] [PubMed] [Google Scholar]


