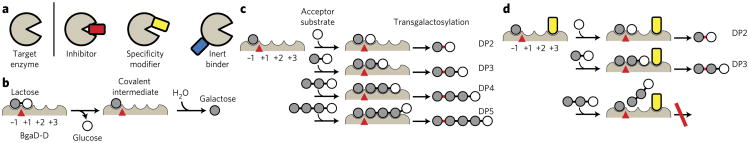
Figure 1. Modulation of enzyme catalytic properties with synthetic binding proteins.
(a) Schematic drawing of different classes of binding proteins. (b–d) Schematic representations of the hydrolysis reaction (b) and of the transgalactosylation reaction of BgaD-D in the absence (c) or the presence (d) of a specificity modifier (yellow). Hypothesized subsites are labeled −1, +1, +2 and +3. The catalytic site where hydrolysis and transgalactosylation reactions occur is shown as a red triangle. In c and d, the initial step of lactose cleavage is omitted, and the reactions from the covalent intermediate are shown for brevity. A sugar linkage newly formed by the transgalactosylation reaction is marked in red. DP2, DP3, DP4 and DP5 denote di-, tri-, tetra- and pentasaccharides, respectively. The specificity modifier restricts the access of larger oligosaccharides to the subsites (d). The inhibition of DP4 production naturally leads to the inhibition of the production of DP5 and larger oligosaccharides (not shown).