Abstract
Fusion between TMPRSS2 and ERG, placing ERG under the control of the TMPRSS2 promoter, is the most frequent genetic alteration in prostate cancer, present in 40–50% of cases. The fusion event is an early, if not initiating, event in prostate cancer, implicating the TMPRSS2-positive prostate epithelial cell as the cancer cell of origin in fusion-positive prostate cancer. To introduce genetic alterations into Tmprss2-positive cells in mice in a temporal-specific manner, we generated a Tmprss2-CreERT2 knock-in mouse. We found robust tamoxifen-dependent Cre activation in the prostate luminal cells but not basal epithelial cells, as well as epithelial cells of the bladder and gastrointestinal (GI) tract. The knock-in allele on the Tmprss2 locus does not noticeably impact prostate, bladder, or gastrointestinal function. Deletion of Pten in Tmprss2-positive cells of adult mice generated neoplasia only in the prostate, while deletion of Apc in these cells generated neoplasia only in the GI tract. These results suggest that this new Tmprss2-CreERT2 mouse model will be a useful resource for genetic studies on prostate and colon.
Introduction
The prostate epithelium is comprised of two distinct cell layers—an outer layer of basal cells in contact with the stroma and an inner layer of secretory luminal cells that produce constituents of the prostatic fluid. Prostate cancer shares many molecular and histologic similarities with luminal cells, including growth dependence on the androgen receptor (AR), as well as AR-dependent expression of seminal fluid proteases PSA and TMPRSS2. In contrast, prostate cancer seldom express basal markers and the absence of basal markers is a pathological criteria to diagnose prostate cancer.
Although most primary prostate cancers have luminal cell histology, the etiology and cell of origin of prostate cancer remain controversial. Early studies based on sphere formation in vitro and graft formation in vivo suggested that basal cells form the stem cells of the normal prostate and are the cells origin of prostate cancer [1,2]. However, recent studies using lineage tracing in genetically engineered mouse (GEM) models support the existence of a stem cell population in both the basal and luminal epithelia of the prostate [3,4] Within the luminal compartment, castration-resistant Nkx3-1-expressing cells (CARNs) have been shown to represent a source of stem cells that can regenerate the prostate epithelium and can be transformed when Pten is deleted [5]. Using the recently developed prostate 3D organoid culture system, we have shown that both single luminal and basal cells from either human or mouse prostates can give rise to organoids, implicating the existence of bipotential stem cells in each compartment [6,7]. Recent evidence also indicated that luminal cells are favored as cells of origin of prostate cancer [8].
Genomic fusion between the membrane-bound serine protease TMPRSS2 and the ETS-family transcription factor ERG is an early genetic alteration occurring in ~50% of prostate cancers [9,10]. The fusion event results in ERG overexpression under the TMPRSS2 promoter. These findings support the notion that TMPRSS2-expressing cells are important for prostate cancer initiation, and genomic alterations of these cells may trigger pathogenetic events. The identity of TMPRSS2-expressing cells is not fully elucidated. Different studies have reported that Tmprss2 is preferentially expressed in basal cells, or luminal cells, or both [11,12,13].
In order to identify Tmprss2 expressing cells, trace their lineage, and determine their tumorigenic capacity, we generated a tamoxifen-inducible knock-in mouse model carrying the CreERT2 gene under the control of the Tmprss2 promoter. We demonstrate the high efficiency of this model to selectively delete genes in the prostate luminal epithelium and colon epithelium. Furthermore, we show that conditional deletion of Pten and Apc in Tmprss2 expressing cells lead to prostate and colorectal transformation, respectively.
Materials and Methods
Generation of the Tmprss2-CreERT2 mouse
All mouse studies are approved by MSKCC Institutional Animal Care and Use Committee under protocol 11-12-027. Institutional guidelines for the proper, humane use of animals in research were followed.
To generate Tmprss2-FRT-NEO-FRT-CreERT2 targeting construct, we started with pRosa26PAm1 (a gift from Douglas Melton, Addgene #15036) [14,15], a targeting plasmid that contains PacI and AscI cloning sites between the 5’ and 3’ homology arms of the Rosa26 locus, followed by a diphtheria toxin cassette (DTA). We replaced the Rosa26 5’ arm with 3.15 kb fragment 5’ of exon 2 of the Tmprss2 gene, generated by PCR using the 5’-TGG CTT CTG CTT CTG ATG-3’ and 5’-GCG TTA ATT AAG CCT TCA GCC TTC ACT TCA C-3’ primer pair on a mouse BAC clone, and cloned using PacI and SacII sites. We then replaced the Rosa26 3’ arm with a 5.05 kb fragment 3’ of exon 2 of the Tmprss2 gene, generated by PCR using the 5’-GGG GCG CGC CTG GCC TTT TCC TTG TTC CT-3’ and 5’-GGG GGT CGA CAT GTG GCT CAG TGG TAA A-3’ primer pair, and cloned using AscI and SalI sites. PCR was performed using PFU turbo (Stratagene). We named the product pTmprss2PAm1.
Next, we took pBTG[14,15] (a gift from Douglas Melton, Addgene #15037), a plasmid with adenovirus splice acceptor (SA), followed by a LOX-STOP-LOX cassette, poly-linker site to insert the gene of interest, and IRES-nlsGFP, all between PacI and AscI sites, and performed the following two changes: 1) replaced LOX-STOP-LOX cassette with a FRT-Neo-FRT cassette (PCR amplified from pEZ-Frt-lox-DT (a gift from Klaus Rajewsky, Addgene #11736)) in the reverse direction and 2) cloned CreERT2 from pCAG-CreERT2 (a gift from Connie Cepko, Addgene #14797)[16] into the poly-linker site. We cloned the Frt-Neo-Frt-SA-CreERT2-IRES-nlsGFP into pTmprss2PAm1 to generate the targeting vector.
Gene targeting was performed at the Rockefeller University Gene Targeting Resource Center (Head: Chingwen Yang). The targeting plasmid was electrophoresed into albino C57BL/6J ES cells and G418 resistant clones were isolated by standard procedures. The clones were screened by Southern blotting using an external 3’ probe generated by PCR primers 5’-GTC ACC CCT CAC TGC ATT TT-3’ and 5’-ATG GAC ACT CCC AGG CTA GA-3’ cut by HindIII which gave a wild-type 7.5kb band and targeted 8.2kb band. Two positive clones were injected into C57BL/6J blastocysts by the MSKCC Mouse Genetics Core Facility (Head: Willie Mark), and chimeras were mated with albino C57BL/6J females. Germline transmission was confirmed in albino offspring using Southern blotting.
To excise the FRT-Neo-FRT cassette, we crossed the Tmprss2-FRT-NEO-FRT-CreERT2 mice with a FlpE-expressing mouse (B6;SJL-Tg(ACTFLPe)9205Dym/J, Jackson Laboratories) and excision was screened by Southern blotting using a 5’ probe generated by PCR primers 5’-GAT GGA GGC ATC TTT TCA CC-3’ and 5’-CCT CGC TGT CCC AAG ATT AC-3’ cut by EcoRI, which gave a wild-type band of 9.5kb, a targeted band of 9.9kb, and a targeted and FRT-Neo-FRT cassette excised band of 8.0kb. For subsequent generations, Tmprss2-CreERT2 mouse genotyping was performed by PCR of genomic DNA using the following primers: TMP2-11878F (5’-GGT GGG CTC TCC TGG CCA CA3’), TMP2-12186R(5’-TGC CAT CCT GCC T GT GTC AGC -3’), Cre-R4 (5’- CTC GTT GCA TCG ACC GGT AA-3’) with a wild-type band of 300 bp and targeted band of 380 bp.
Mouse alleles
ApcLoxP (APCtm2Rak) mice where exon 14 of Apc is flanked by LoxP sites [17] was a generous gift from Dr. Scott Lowe. Genotyping was performed using primers 5’-CAG ATG TCT TTA TGA GTT TGA-3’ and 5’-AGT GCT GTT TCT ATG AGT CAA C-3’ with a wild-type product of 388bp and LoxP allele of 498bp. To assess Apc deletion in tissue, we performed PCR on genomic DNA isolated from tissues using primers 5’-CAG ATG TCT TTA TGA GTT TGA-3’; 5’-AGT GCT GTT TCT ATG AGT CAA C-3’ and 5’-TTG GCA GAC TGT GTA TAT AAG C; the Apc LoxP and Apc deleted products were 498bp and 568 bp. PtenLoxP mice (Ptentm2.1Ppp) in which exons 4–5 are flanked by LoxP sites [18], were used as previously described [10]. Ai3 (B6.Cg-Gt(ROSA)26Sortm3(CAG-EYFP)Hze/J) mice of conditional CAG-driven YFP expression [19] and mT/mG (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) mice of membrane-targeted tdTomato and EGFP [20] and Actb-FlpE (Tg(ACTFLPe)9205Dym/J) that express the FlpE recombinase under the beta-actin promoter [21] were purchased from Jackson Laboratories.
Mice tamoxifen treatment, sacrifice and tissue analysis
TY, TP and TA mice were injected with tamoxifen at 4mg/40g body weight using a 27-gauge needle and injected once every other day, a total of 3 times. Mice are euthanized by carbon dioxide asphyxiation as recommended by MSKCC Institutional Animal Care and Use Committee under protocol 11-12-027.
For histology and IHC, tissue was harvested from 10-week-old TY males, 22-week-old TP males and 12-week-old TA males. Tissues were fixed in 4% paraformaldehyde (w/v) at 4 degrees Celsius for 12 hours. Tissues were washed three times with cold PBS. All immunohistochemical and histological analyses were conducted by the MSKCC Molecular Cytology Core.
For immunofluorescence, tissues were fixed in 4% paraformaldehyde (w/v) at 4 degrees Celsius for 2 hours. Tissues were washed three times with cold PBS, cryopreserved by overnight incubation in 30% sucrose (w/w), frozen in OCT (Tissue Tek, Sakura Finetek) and sectioned.
FACS and Quantive-RT-PCR
Single cell suspensions of TY mice anterior prostate were stained using CD326-PE/Cy7 (Biolegend; Clone# G8.8; 1:500) and DAPI. All the Q-PCR primers were purchased from Qiagen:
P63: PPM03458A-200
Ck5:PPM59967F-200
Ck14:PPM04519A-200
Ck8:PPM05184A-200
Ck18: PPM05184A-200
Antibodies
The antibodies used for immunohistochemistry were pAKT Ser473 (Cell Signaling Technology; 4060; 1:50 dilution), PTEN (Cell Signaling Technology; 9188; 1:50 dilution), Beta-Catenin (BD Transduction Laboratories; 610154; 1:1000), Ki67 (Abcam; ab16667; 1:100) and GFP (Abcam; ab13970; 1:1000). P63 (Abcam; ab124762; 1:250) and Ck8 (Covance; MMS-162P; 1:1000) were used for immunofluorescence.
Data mining of TMPRSS2 expression
We obtained RNA-seq based TMPRSS2 expression of human tissues from Genotype-Tissue Expression (GTEx) at www.gtexportal.org [22]. We obtained Arrymetrix MOE430 microarray based Tmprss2 expression of mouse tissues from BioGPS [23].
Results
In order to define Tmprss2-expressing cells in prostate, we generated an inducible CreERT2-IRES-nlsGFP mouse model under the control of the mouse Tmprss2 gene promoter. At baseline levels, nuclear GFP expression marks Tmprss2-expressing cells and the Tmprss2-positive lineage can be traced and genetically manipulated when crossed with LoxP-lines and exposed to tamoxifen.
In human prostate cancer, the first exon of TMPRSS2 is entirely within the non-coding 5’ UTR and most TMPRSS2-ERG fusions involve the first intron of TMPRSS2, with the resulting fusion transcript containing no coding sequence of TMPRSS2. We therefore replaced exon 2 with a cassette including an adenovirus splice acceptor (SA), followed by a PGK-driven neomycin selection cassette flanked by FRT recombination sites, followed by the CreERT2-IRES-nlsGFP. After excision of the neomycin cassette, the mouse should express a chimeric transcript containing exon1 of Tmprss2 as the 5’ UTR followed by CreERT2 cDNA (Fig 1A).
Fig 1. Generation of the Tmprss2-CreERT2 knock-in mouse.
(a) Schematic of targeting strategy. A cassette including an adenovirus splice acceptor (SA), followed by PGK-driven neomycin selection cassette flanked by FRT recombination sites, followed by the CreERT2-IRES-nlsEGFP was used to replace exon 2 of mouse Tmprss2. The cassette is flanked by 3.5kb 5’ and 5kb 3’ homology arms. 5’ and 3’ Southern probes as was as HindIII (H) and EcoRI (E) sites and genotyping PCR primers (universal F1, wild-type specific R1, and knock-in specific R2) are depicted. The final transcript includes the non-coding exon 1 of Tmprss2 spliced into the CreERT2-IRES-nlsEGFP gene. (b) Southern blot using 3’ probe and HindIII digestion. WT mice give a 7.5 kb band and the targeted mice (regardless of neomycin cassette) give a 8.2 kb band. (c) Southern blot using 5’ probe and EcoRI digestion. WT mice give a 9.5 kb band; the targeted mice with neomycin cassette (T) give a 9.9 kb band, while the mice with excised neomycin cassette (E) give a 9.5 kb band. (d) Genotype determination of wild-type and heterozygous mice by PCR. Wild-type fragment is 300-bp and mutant is 380-bp.
After homologous recombination in embryonic stem cells and germline transmission, we observed two independent lines with the correct integration verified by Southern blot analysis using both 5’ and 3’ probes (Fig 1B). We next crossed F1 mice with Actb-FlpE mice and Southern blot analysis using the 5’ probe confirmed excision of the neomycin cassette in all FlpE and CreERT2 double-positive F2 mice (Fig 1C). Genotyping for the Tmprss2-CreERT2 allele was performed by polymerase chain reaction (PCR), resulting in a 380-bp product for the knock-in allele and a 300-bp product for wild-type (Fig 1A and 1D). Homozygous Tmprss2-CreERT2 had no visible phenotype and were generated at Mendelian ratio, consistent with prior observation that Tmprss2 knockout mice had no phenotype [24].
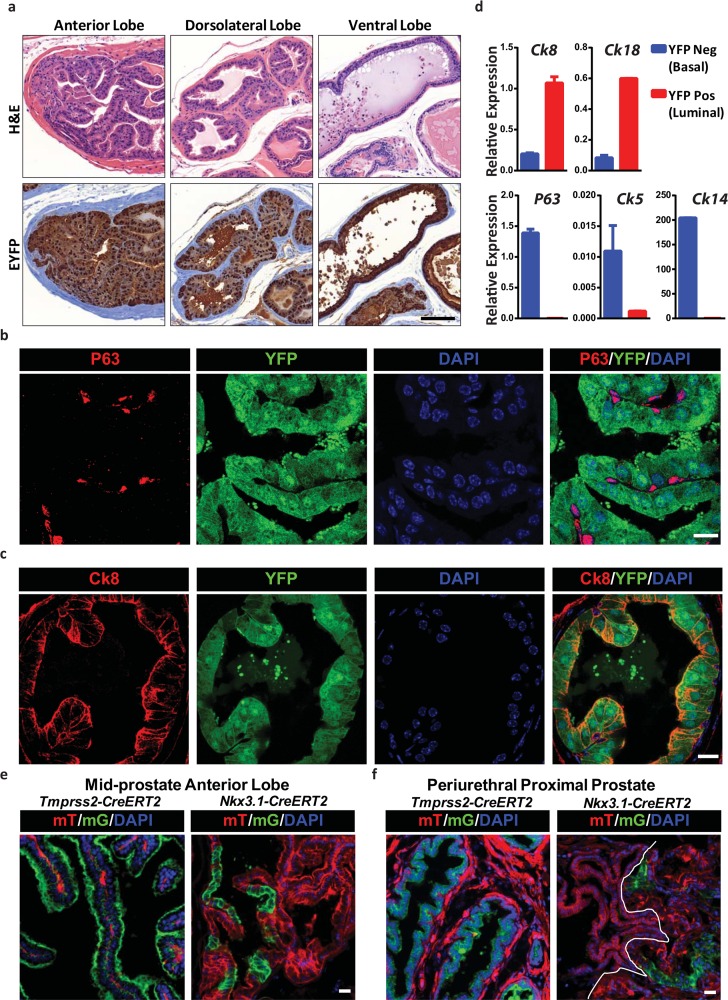
The nuclear GFP signal of CreERT2-IRES-nlsGFP is dim at baseline levels and is difficult for direct lineage trace. To test the efficiency and specificity of the novel knock-in line, we crossed Tmprss2-CreERT2 mice with Rosa26-EYFP mice with a CAG driven YFP Cre-reporter [19]. We examined the YFP expression pattern in the prostate gland of 10-week-old male Tmprss2-CreERT2; Rosa26-EYFP /EYFP (TY) mice after 2 weeks of tamoxifen treatment. YFP immunohistochemistry (IHC) showed that labeling was highly efficient, and the majority of the prostate epithelium appeared labeled (Fig 2A). Analysis of direct YFP fluorescence combined with immunofluorescence (IF) against luminal marker Cytokeratin 8 (Ck8) or basal maker p63 showed that only the Ck8 positive luminal cells exhibited YFP signal, while the p63 positive basal cells and stroma cells were negative for YFP in the anterior, dorsolateral and ventral lobes of the prostate (Fig 2B and 2C). Quantification of YFP-expressing cells indicated that in the anterior and dorsolateral lobes, approaching 100% of luminal epithelial cells (n = 635) were positive and in the ventral lobe, approximately 80% of luminal cells (n = 476) were positive. We separated YFP-positive and YFP-negative prostate epithelia cells from TY mice anterior prostate tissue using florescence activated cell sorting (FACS). Consistent with our histological observation, the YFP-positive population had high luminal-cell-specific gene expression, Ck8 and Ck18, while the YFP-negative population had high basal-cell-specific gene expression, P63, Ck5 and Ck14 (Fig 2D).We compared the Tmprss2-CreERT2 recombinase activity with that of Nkx3-1-CreERT2, a commonly used inducible Cre-driver for prostate luminal cells. We crossed both lines to Rosa26-mT/mG, a mouse that expresses membrane-targeted tdTomato (mT) at baseline- and membrane-targeted GFP (mG) after excision [20] and found two important differences that highlight the utility of the Tmprss2-CreERT2 mouse model. First, the efficiency of recombination within prostate luminal cells is much higher in Tmprss2-CreERT2 (99.6%, n = 234) compared to Nkx3-1-CreERT2 (23.7%, n = 223). Second, within the proximal peri-urethral prostate, which is comprised of more compact epithelial cells and is thought to have a greater number of stem cells, Tmprss2-CreERT2 is highly active (97.7%, n = 215), whereas Nkx3-1-CreERT2 lacks activity (1.04%, n = 192) (Fig 2E and 2F).
Fig 2. Prostate specific activity of Tmprss2-CreERT2 mice.
(a) H&E and YFP IHC of anterior prostate, dorsolateral prostate, ventral prostate in TY mice. Scale bar represents 100 μM. (b) IF stain of basal cell marker P63 with endogenous YFP and DAPI fluorescence in TY mice. Scale bar represents 20 μM. (c) IF stain of luminal cell marker Ck8 with endogenous YFP and DAPI fluorescence in TY mice. Scale bar represents 20 μM. (d) Quantitative RT-PCR analysis of basal (Ck5, Ck14, P63) and luminal (Ck8, Ck18) marker expression in YFP+ and YFP- epithelial cells. Basal cell markers are strongly expressed YFP- cells; luminal cell markers strongly expressed in YFP+ cells. Expression was normalized to Actin. Results are shown as mean ± SD. (e) Comparison of Tmprss2-CreERT2 with Nkx3.1-CreERT2 driven conversion of membrane tdTomato (mT) to membrane EGFP (mG) of the anterior prostate. Scale bar represents 50 μM. (f) Same as in (e) but in periurethral proximal prostate. These cells are tightly packed with scant cytoplasm. The white line in the Nkx3.1-CreERT2 mouse separates the anterior prostate from periurethral prostate. Scale bar represents 50 μM.
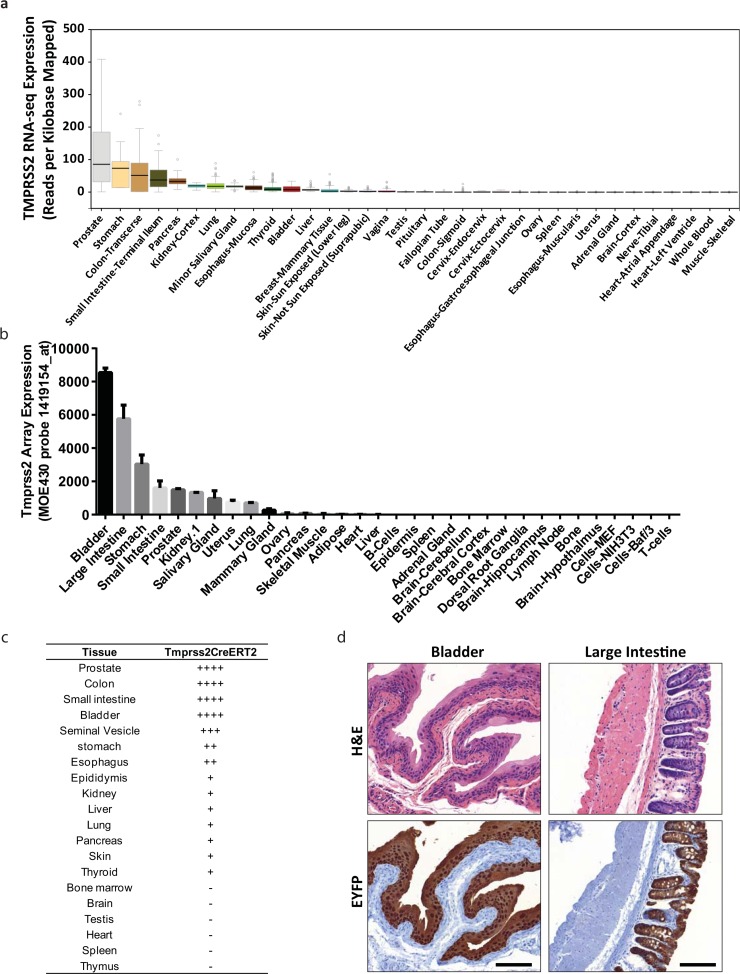
Mining of normal tissue gene expression data showed that TMPRSS2 is not prostate-specific and is expressed in multiple organs, specifically of the GI tract in both human and mouse (Fig 3A and 3B). Notably, TMPRSS2 expression also have difference between human and mouse, such as bladder, which has relatively high expression in mouse, but it appears to be quite low in human. To determine the tissue specificity of CreERT2 activity beyond the prostate, nineteen other tissues, namely the testis, epididymis, seminal vesicle, pancreas, colon, small intestine, stomach, esophagus, bladder, bone marrow, brain, heart, kidney, liver, lung, skin, spleen, thymus and thyroid were collected from male TY mice and analyzed for YFP staining. We found that almost all the epithelial cells of colon and bladder were positive for YFP staining, while there were no detectable YFP positive stroma cells in these organs (Fig 3C and 3D). Recombination was also observed in epididymis, seminal vesicle, pancreas, small intestine, stomach, esophagus, kidney, liver, lung, skin and thyroid (Fig 3C).
Fig 3. Tissue distribution of Tmprss2-CreERT2 mediated recombination.
(a) RNA-seq-based expression of Tmprss2 mRNA in human tissues from the Genotype-Tissue Expression (GTEx) project. (b) MOE430-based expression of Tmprss2 mRNA in mouse tissues from BioGPS. (c) Table of recombination efficiency, based on YFP IHC, in tissues of TY mice after tamoxifen administration. (d) H&E and YFP IHC of bladder and colon in TY mice. Scale bars represent 100 μM.
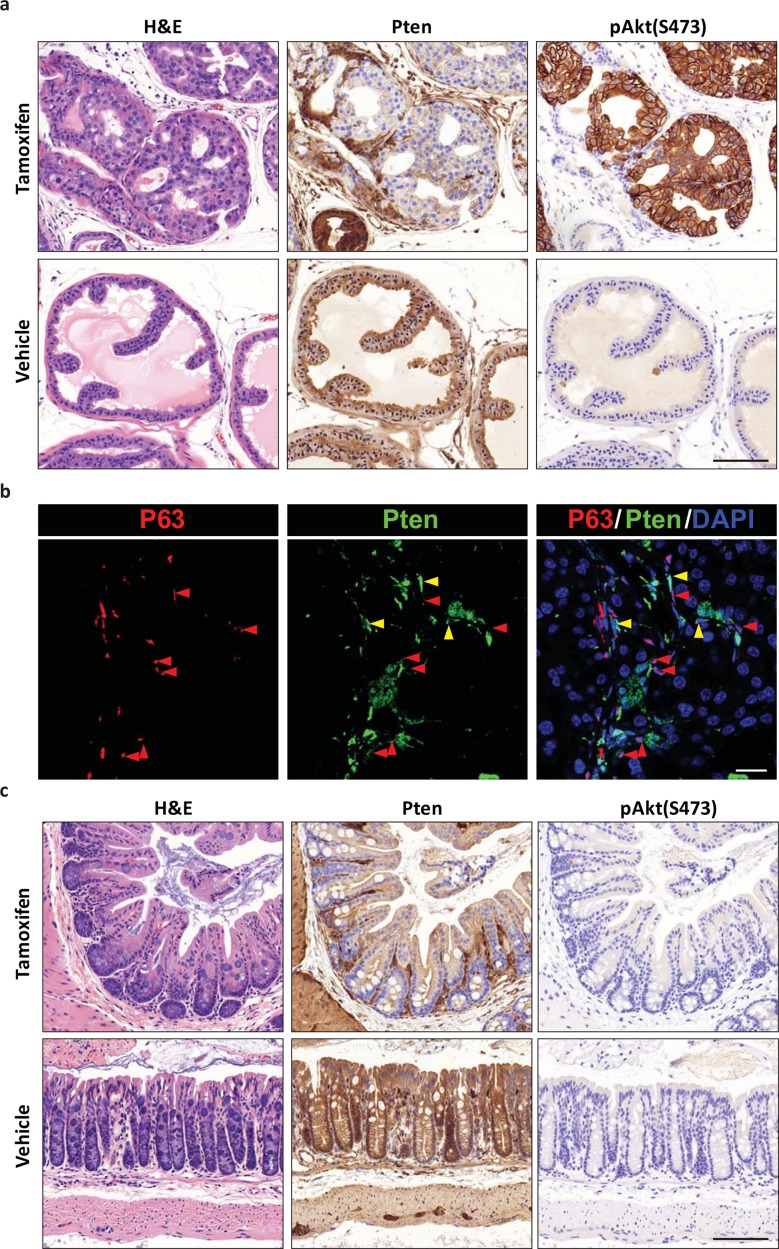
We asked whether Tmprss2-CreERT2 is active in the tumor initiating cells and if Tmprss2-CreERT2-mediated deletion of tumor suppressor genes can generate tissue specific tumorigenesis. First, we analyzed deletion of Pten, a tumor suppressor implicated in prostate cancer initiation. To achieve Pten deletion, we crossed PtenLoxP/LoxP mice [18] to the Tmprss2-CreERT2 knock-in line. We treated the Tmprss2-CreERT2/ T2; PtenLoxP/LoxP (TP) mice with tamoxifen at 8 weeks of age and analyzed the prostate and colon 12 weeks later. Hematoxylin and eosin (H&E) staining of the prostate showed prevalent prostatic intraepithelial neoplasia (mPin), with cribriform growth and enlarged nuclei in tamoxifen but not vehicle injected mice (Fig 4A). IHC and IF staining of PTEN and P63 showed that Pten loss is specific to the prostate luminal epithelial cells, while Pten is expressed in some prostate basal epithelial cells and stromal cells surrounding the prostate acini (Fig 4A and 4B). The loss of Pten corresponds with an increase of AKT phosphorylation in tamoxifen treated prostate luminal cells. We next analyzed colonic epithelium in these TP mice and found no visible gross or histological abnormalities. IHC analysis showed robust loss of Pten throughout the colonic epithelium. However, AKT phosphorylation was not detectable using the same staining method as the prostate pAKT staining, suggesting very low baseline PI-3 kinase activity that cannot induce AKT phosphorylation despite loss of Pten (Fig 4C).
Fig 4. Tissue-specific tumorigenesis of Pten deletion.
(a) H&E and Pten, pAkt(S473) IHC of prostate from TP mouse following tamoxifen or vehicle administration. Scale bar represents 100 μM. (b) IF stain of basal cell marker P63 with Pten and DAPI fluorescence in TP mice. Red arrows and yellow arrows indicate P63+ basal cell and P63- stromal cells. Scale bar represents 25 μM. (c) H&E and Pten, pAkt(S473) IHC of prostate from TP mouse following tamoxifen or vehicle administration. Scale bar represents 100 μM.
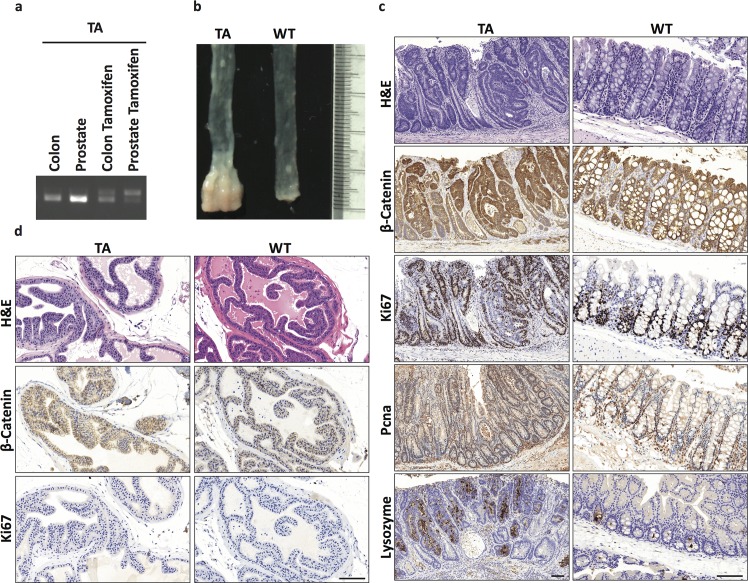
We next analyzed deletion of Apc, a gatekeeper in colorectal cancer tumorigenesis. We crossed ApcLoxP/LoxP mice to the Tmprss2-CreERT2 mice and treated the Tmprss2-CreERT2/T2; ApcLoxP/LoxP (TA) mice with tamoxifen at 8 weeks of age and analyzed the mice colon and prostate 12 weeks post tamoxifen treatment. PCR of genomic DNA isolated from prostate and colon showed the presence of PCR product specific for Apc-deletion only in tamoxifen-treated mice (Fig 5A). The colonic epithelium of tamoxifen treated TA mice showed features characteristic of Apc-loss adenomas (Fig 5B). The loss of Apc in these mice corresponds to an increase in β-catenin staining and marked hyperproliferation with increased Ki67 and Pcna staining outside the crypts, characteristic of differentiation arrest by the identification of lysozyme-positive Paneth cells outside the base of crypt (Fig 5C). Although the prostate had loss of Apc (Fig 5A), the prostate exhibited normal histology (Fig 5D). IHC showed a subtle increase in membrane-localized β-catenin levels in cells and no change in Ki67 index (Fig 5D).
Fig 5. Tissue specific tumorigenesis of Apc deletion.
(a) Characterization of Cre-mediated recombination in Tam-treated Tmprss2CreERT2/ T2; ApcLoxP/LoxP (TA) mice. Genomic DNA isolated from the indicated organs of a Tam-treated mouse were analyzed by PCR. The positions of the Apc floxed 498bp, and recombined 568bp DNA segments are indicated. (b) Gross pathology of colon of TA and wild-type mice following tamoxifen administration showing macroscopic colon polyps in tamoxifen treated mice. (c) H&E and β-catenin, Ki67 stain of TA and wild-type mouse colon. (d) H&E and β-catenin, Ki67 stain of TA and wild-type mouse prostate. Scale bars represent 100 μM.
Discussion
GEM models of cancer have provided important insights into cancer initiation and progression, and have important implications for clinical studies and clinical trials. Various mouse lines were generated over recent years by selectively introducing targeted mutations in prostatic epithelium through Cre recombinase under the control of the mouse mammary tumor virus promoter MMTV-Cre [25], a modified probasin promoter PB-Cre4 [26], or the prostate-specific antigen (PSA) promoter PSA-Cre [27,28]. More recently, several prostate-specific CreERT2 mouse models, including knock-in to the Nkx3-1 locus [5], transgenic under the human PSA promoter [29] and transgenic under a modified probasin promoter (ARR2Pb) [30] have been generated, each able to induce mPin when crossed to PtenLoxP/LoxP mice. This Tmprss2-CreERT2 knock-in strain is highly efficient and allows precise control over the timing of introducing gene alterations in the mouse prostate and colon, and accurately mimics the late onset of human prostate cancer and colon cancer.
Here, we show that Tmprss2-CreERT2 is expressed in several organs and rapidly induces prostate neoplasia after induction of Pten deletion and colorectal neoplasia after induction of Apc deletion. We further find that the effects of oncogenic activation are surprisingly tissue-specific and recapitulate that of human cancer. In human prostate cancer, PTEN loss is an early tumorigenic lesion, while Wnt-pathway activation through aberrations in APC, CTNNB1, RNF43, and RSPO2 are found in metastatic castration-resistant cancer, suggesting they are associated with tumor progression [31,32]. In human colorectal cancer, Wnt-pathway activation is a gatekeeper event while mutations of loss of PTEN rarely occur.
In conclusion, we have established a new CreERT2 knock-in mouse line, and these knock-in mice can be used to investigate Tmprss2-expressing cells and their descendant cells at various stages in vivo. We believe that the generated knock-in mice in this article could be useful for studying the initiation and progression of prostate and colon cancers.
Acknowledgments
We would like to thank the following core facilities: MSKCC Molecular Cytology (Ning Fan and Mesruh Turkekul), MSKCC Mouse Genetics (Willie Mark, Lorena Osorio, Alison Bromberg, Jacquelyn Song, Mayumi Isaka, Peter Romanienko), Rockefeller University Gene Targeting Resource Center (Chingwen Yang).
Data Availability
All relevant data are within the paper.
Funding Statement
This work is supported in part by NYSTEM Stipend (C026879) to DG, the Howard Hughes Medical Institute (CLS), NCI (K08CA140946, YC; R01CA193837, CLS, YC; P50CA092629, CLS, YC; K08CA151660, PC; DP2 CA174499, PC; U01 CA141502, CLS), US DOD (W81XWH-10-1-0197), Prostate Cancer Foundation (YC), Starr Cancer Consortium (YC, PC, CLS), Geoffrey Beene Cancer Research Center (YC, PC), Gerstner Family Foundation (YC), Bressler Scholars Fund (YC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON (2010) Identification of a cell of origin for human prostate cancer. Science 329: 568–571. 10.1126/science.1189992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xin L, Lawson DA, Witte ON (2005) The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A 102: 6942–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi N., Zhang B., Zhang L., Ittmann M., and Xin L. (2012). Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21, 253–265. 10.1016/j.ccr.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parisotto M., and Metzger D. (2013). Genetically engineered mouse models of prostate cancer. Mol Oncol 7, 190–205. 10.1016/j.molonc.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, et al. (2009) A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461: 495–500. 10.1038/nature08361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, et al. (2014) Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159: 163–175. 10.1016/j.cell.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, et al. (2014) Organoid cultures derived from patients with advanced prostate cancer. Cell 159: 176–187. 10.1016/j.cell.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ZA, Toivanen R, Bergren SK, Chambon P, Shen MM (2014) Luminal cells are favored as the cell of origin for prostate cancer. Cell Rep 8: 1339–1346. 10.1016/j.celrep.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310: 644–648. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Chi P, Rockowitz S, Iaquinta PJ, Shamu T, Shukla S, et al. (2013) ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat Med 19: 1023–1029. 10.1038/nm.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD, et al. (1999) Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res 59: 4180–4184. [PubMed] [Google Scholar]
- 12.Casey OM, Fang L, Hynes PG, Abou-Kheir WG, Martin PL, Tillman HS, et al. (2012) TMPRSS2- Driven ERG Expression In Vivo Increases Self-Renewal and Maintains Expression in a Castration Resistant Subpopulation. PLoS One 7: e41668 10.1371/journal.pone.0041668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YW, Lee MS, Lucht A, Chou FP, Huang W, Havighurst TC, et al. (2010) TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol 176: 2986–2996. 10.2353/ajpath.2010.090665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology 1: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA (2003) Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100: 14920–14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda T, Cepko CL (2007) Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci U S A 104: 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuraguchi M, Wang XP, Bronson RT, Rothenberg R, Ohene-Baah NY, Lund JJ, et al. (2006) Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet 2: e146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, et al. (2003) Pten dose dictates cancer progression in the prostate. PLoS Biol 1: E59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L (2007) A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, et al. (2000) High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25: 139–140. [DOI] [PubMed] [Google Scholar]
- 22.Consortium GT (2013) The Genotype-Tissue Expression (GTEx) project. Nat Genet 45: 580–585. 10.1038/ng.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, et al. (2009) BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome biology 10: R130 10.1186/gb-2009-10-11-r130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TS, Heinlein C, Hackman RC, Nelson PS (2006) Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Mol Cell Biol 26: 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner K.U., McAllister K., Ward T., Davis B., Wiseman R., and Hennighausen L. (2001). Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res 10, 545–553. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, et al. (2001) Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 101: 61–69. [DOI] [PubMed] [Google Scholar]
- 27.Abdulkadir SA, Magee JA, Peters TJ, Kaleem Z, Naughton CK, Humphrey PA, et al. (2002) Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol 22: 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma X, Ziel-van der Made AC, Autar B, van der Korput HA, Vermeij M, van Duijn P, et al. (2005) Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res 65: 5730–5739. [DOI] [PubMed] [Google Scholar]
- 29.Ratnacaram CK, Teletin M, Jiang M, Meng X, Chambon P, Metzger D (2008) Temporally controlled ablation of PTEN in adult mouse prostate epithelium generates a model of invasive prostatic adenocarcinoma. Proc Natl Acad Sci U S A 105: 2521–2526. 10.1073/pnas.0712021105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luchman HA, Benediktsson H, Villemaire ML, Peterson AC, Jirik FR (2008) The pace of prostatic intraepithelial neoplasia development is determined by the timing of Pten tumor suppressor gene excision. PLoS One 3: e3940 10.1371/journal.pone.0003940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. (2015) Integrative clinical genomics of advanced prostate cancer. Cell 161: 1215–1228. 10.1016/j.cell.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Research N (2015) The Molecular Taxonomy of Primary Prostate Cancer. Cell 163: 1011–1025. 10.1016/j.cell.2015.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.