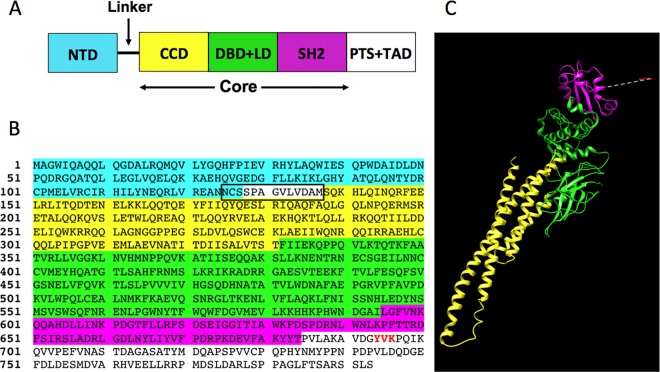
Fig 1. The domain structure of STAT proteins.
(A) Names and positions of the six domains on the primary sequence: NTD (N-terminal domain), CCD (4-helix bundle coiled-coil domain), DBD (DNA-binding domain), LD (linker or connector domain), SH2, and TAD (transactivation domain). We treated the DBD and LD as one combined domain, as SCOP [17] does for STAT3β. There is a short flexible 13-residue linker between the NTD and CCD, which partly overlaps with the NTD. There is also a phospho-tyrosine containing segment (PTS) between the SH2 and TAD, which we combined with the TAD. See Methods for more detail. (B) The sequence of mouse STAT5A (NCBI Accn# CAA88419.1). The residues are highlighted according to the domains to which they belong, using the same coloring scheme used in (A). The 13-residue linker is boxed. The phospho-tyrosine residue (Y694) and the two following residues (V695 and K696) are shown in red. (C) STAT5A domains in the modeled structure of the STAT5A core, which includes the CCD, DBD, LD, SH2 and the residues 694–696 of the PTS. Dotted line represents the connecting residues (685–693) that were not included in the model.