Figure 3.
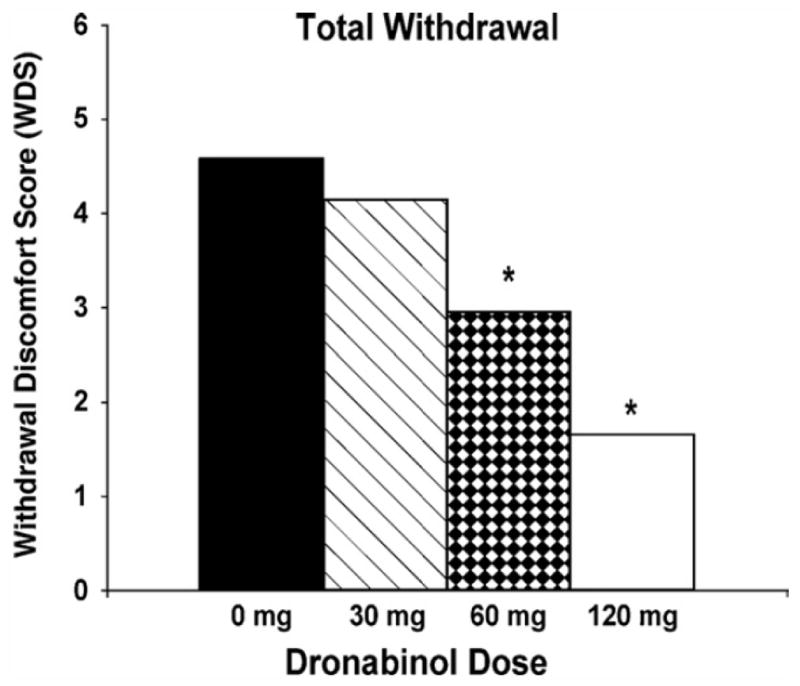
Effects of treatment with dronabinol on cannabis withdrawal symptoms in daily cannabis users. Significant withdrawal symptoms were observed during the placebo maintenance phase for subjective ratings of decreased appetite, diarrhea, nausea, stomach pain, irritability, sleep difficulty, total sleep time, subjective sleep quality, mood at morning awakening, alertness at morning awakening, restlessness, nervousness/anxiety, chills, increased aggression, increased anger, headaches, difficulty concentrating, and total withdrawal discomfort score (WDS). Significant dose-dependent effects of dronabinol were observed for each withdrawal item except “sleep difficulty” and “nervousness/anxiety.” Mean subjective withdrawal ratings (WDS) are presented as a function of dronabinol maintenance dose. Asterisks indicate statistically significant differences between dronabinol dose conditions and placebo. These results suggest that replacement therapy with a CB1 receptor agonist can decrease withdrawal symptoms, analogous to nicotine replacement therapy for tobacco smokers. Figure from Vandrey, R., Stitzer, M. L., Mintzer, M. Z., Huestis, M. A., Murray, J. A., & Lee, D. (2013). The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug and Alcohol Dependence, 128(1–2), 64–70. doi:10.1016/j.drugalcdep.2012.08.001 with permission.
