Abstract
Rett Syndrome (RTT) is a neurodevelopmental disorder affecting multiple functions, including the norepinephrine (NE) system. In the CNS, NE is mostly produced by neurons in the locus coeruleus (LC) where defects in intrinsic neuronal properties, NE biosynthetic enzymes, neuronal CO2 sensitivity and synaptic currents have been reported in mouse models of RTT. The LC neurons in Mecp2-null mice show a high rate of spontaneous firing, while whether such hyperexcitability may increase or decrease the NE release from synapses is unknown. To activate the NE-ergic axonal terminals selectively, we generated an optogenetic mouse model of RTT where NE-ergic neuronal excitability can be manipulated with light. Using commercially available mouse breeders, we produced a new strain of double transgenic mice with Mecp2 knockout and channelrhodopsin knock-in in catecholaminergic neurons. Several RTT-like phenotypes were found in the TH-ChR-Mecp2−/Y mice, including hypoactivity, low body weight, hind limb clasping, and breathing disorders. In brain slices, optostimulation produced depolarization and an increase in the firing rate of LC neurons from TH-ChR control mice. Optostimulation of presynaptic NE-ergic neurons augmented the firing rate of hypoglossal neurons in TH-ChR control mice, which was blocked by the α-adrenoceptor antagonist phentolamine. Such optostimulation of NE-ergic terminals had barely any effects on hypoglossal neurons from two to three TH-ChR-Mecp2−/Y mice, indicating that excessive excitation of presynaptic neurons does not benefit NE-ergic modulation in mice with Mecp2 disruption. Also, these results demonstrate the feasibility to generate double transgenic mice for studies of RTT using commercially available mice, which are inexpensive, labor / time-efficient, and promising for cell-specific stimulation.
Keywords: Mecp2, Rett syndrome, optogenetics, double transgenic, NE modulation, IMR_JAX:008601, IMSR_JAX:012569, IMSR_JAX:003890, AB_10055152, AB_2566814, AB_10049285, AB_258613, AB_2566815, SCR_011323
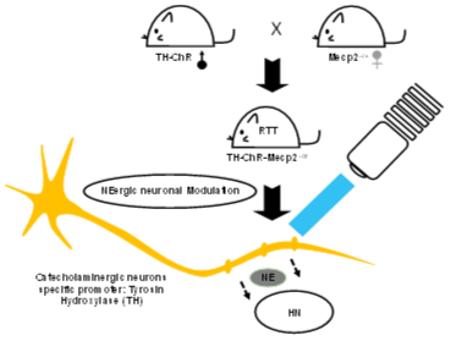
Graphic Abstract
The compensatory relationship between NE deficiency and the LC neuron hyperexcitability in RTT has been unclear. We generated a double transgenic mice model to answer this question that carries Mecp2 disruption while allowing specific intervention to NE-ergic neurons, TH-ChR-Mecp2−/Y. With several RTT-like phenotypes, it is feasible to use this model to study the neuronal modulation in RTT.
Introduction
Rett Syndrome (RTT) is a neurodevelopmental disorder seen in 1 of every 10,000 female births. The disease affects multiple functions and systems including the norepinephrine (NE) system. As a result of the NE defect, people with RTT have breathing abnormalities and other autonomic dysfunctions that are also found in mouse models of RTT with Mecp2 disruption (Julu et al. 2001). In the CNS, NE is mainly produced by neurons in the locus coeruleus (LC). These NE-ergic neurons in the LC project to the frontal cortex, hippocampus, cerebellum, spinal cord and multiple brainstem nuclei, brain regions that are involved in regulation of cognition, attention, anxiety, psychosis, motor control, and cardiorespiratory functions. Several previous studies have shown that LC neurons in Mecp2−/Y mice have decreased NE biosynthesis enzymes, defective intrinsic membrane properties, insufficient GABA synaptic inhibition, and increased excitability (Zhang et al. 2010; Zhong et al. 2015).
The NE content in the brainstem and whole-brain preparations is markedly reduced in Mecp2−/Y mice compared to the WT mice (Ide et al. 2005; Viemari et al. 2005). The NE metabolite 3-methoxy-4-hydroxyphenylethylene (MHPG) is low in the cerebrospinal fluid of RTT patients (Zoghbi et al. 1985). The decreases in NE content and NE metabolites occur earlier and appear more severe than changes in other neurotransmitters (Zoghbi et al. 1989). Application of exogenous NE or NE uptake inhibitors improves respiratory rhythm, increases the number of TH-positive neurons in the medulla, and extends the lifespan of Mecp2−/Y mice (Roux et al. 2007; Villard and Roux 2006; Zhang et al. 2011).
The defect in the NE system is associated with LC neuronal hyperexcitability or the increased firing rate, while their relationship is unclear. An increase in LC neuronal firing activity would theoretically enhance the NE release from presynaptic terminals, whereas the poor expression of TH and DBH may reduce NE biosynthesis in LC neurons hindering the effect of neuronal excitability. Although the increased LC neuronal excitability may be a compensatory response of LC neurons to the deficiency in NE production, whether such a process is indeed beneficial to the NE-ergic modulation remains to be demonstrated. The understanding of these problems relies on an animal model that carries the Mecp2 defect and allows an access to the NE-ergic neuronal excitability. Therefore, we generate a new optogenetic mouse model of RTT by targeting on catecholaminergic neurons using commercially available mouse breeders, characterized several RTT-like phenotypes, and studied the NE-ergic neuronal modulation in brain slices.
Methods and Materials
Animals breeding and genotyping
All procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Georgia State University Institutional Animal Care and Use Committee. The following mouse lines were used for the breeding of double transgenic mice: TH::IRES-cre (Jackson Laboratory, stock number: 008601, strain name: B6.Cg-Tg(Th-cre)1Tmd/J, RRID:IMR_JAX:008601 (Lindeberg et al. 2004), ChR-loxP, which is ChR-eYFP downstream of a floxed STOP cassette (Jackson Laboratory, stock number: 012569, strain name: B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J, RRID:IMSR_JAX:012569) (Madisen et al. 2012), female heterozygous Mecp2+/− mice (Jackson Laboratory, stock number 003890, strain name: B6.129P2(C)-Mecp2tm1.1Bird/J, RRID:IMSR_JAX:003890) (Guy et al. 2001). The TH::IRES-cre mice were cross-bred with the ChR-loxP mice to produce single transgenic TH-ChR mice. After that, the TH-ChR mice were cross-bred with female Mecp2+/− mice to generate double transgenic TH-ChR-Mecp2−/Y. All experiments were performed in double transgenic hemizygous Mecp2-null males (TH-ChR-Mecp2−/Y) and control males (TH-ChR). Genotyping was performed according to the primer information provided by Jackson Laboratory.
Both double transgenic mice, TH-ChR-Mecp2−/Y, mice (n=11 animals) and littermate control TH-ChR mice (n=11 animals) were weighted during age four to nine weeks old. The hindlimb clasping photo of both strains mice were taken at five weeks postnatally (see below).
Immunohistochemistry
Double transgenic mice, TH-ChR-Mecp2−/Y, at 7 weeks of age were anesthetized with inhalation of saturated isoflurane and transcardially perfused with 0.9% saline and 4% (w/v) paraformaldehyde sequentially. Then the brain was removed, and the pontine transverse sections (30-40 μm) were cut from the brainstem on a cryostat (Leica, Wetzlar, Germany). Catecholaminergic neurons in both LC and medullary areas were labelled with anti-dopamine beta-hydroxylase (DBH, red) and anti-GFP (green) antibodies that also detect YFP. Briefly, the frozen sections were incubated with primary GFP/YFP antibodies (mouse monoclonal, 1:400, life technologies, RRID:AB_10055152) and anti-DBH (rabbit polyclonal, 1:1000, Sigma, RRID:AB_2566814), followed by AF488 conjugate donkey anti-mouse (polyclonal, 1:400, life technologies, RRID:AB_10049285) and biotin conjugate goat anti-rabbit (polyclonal, 1:400, Sigma, RRID:AB_258613) secondary antibodies. Finally, the slides were treated with Texas Red-conjugated antibody (1:400, Jackson ImmunoResearch, RRID:AB_2566815), followed with dehydration. Antibodies used in the experiments are listed in Table I. After mounted with 2,2'-Thiodiethanol (Sigma), the fluorescence images were taken with an LSM 510 Zeiss confocal microscope (Jena, Germany).
Table I.
Antibodies
| Antigen | Description of immunogen | Source, host species, catalog No., RRID |
Dilution |
|---|---|---|---|
| DBH (primary) | recombinant fragment corresponding to a region within amino acids 8 and 320 of Dopamine beta Hydroxylase |
Sigma, rabbit polyclonal, SAB2701977, RRID:AB_2566814 |
1:1000 |
| GFP (primary) | Life Technologies, mouse monoclonal, A11120, RRID:AB_10055152 |
1:400 | |
| Alexa Fluor 488- conjugated donkey anti- mouse IgG (H+L) |
Life Technologies, Donkey polyclonal, A21202, RRID:AB_10049285 |
1:400 | |
| Anti-Mouse IgG (whole molecule)–Biotin antibody |
Sigma, goat polyclonal, B8774, RRID:AB_258613 |
1:400 | |
| Texas, Red-conjugated Donkey Anti-Mouse IgG (H+L) |
Jackson ImmunoResearch, donkey polyclonal, RRID:AB_2566815 |
1:400 |
Plethysmograph
Breathing activity was recorded from conscious mice of both double transgenic TH-ChR-Mecp2−/Y mice (n=10 animals) and TH-ChR mice (n=9 animals) at age 4-5 weeks old without anesthesia. The mice were kept in a plethysmograph chamber (~ 40 ml) with a same volume reference chamber connected to the same gas source. Mouse breathing signals were barometrically recorded continuously by measuring the pressure changes between the animal chamber and a reference chamber with a force-electricity transducer (PanaVise Products, Inc. Nevada). The chambers were constantly ventilated with air at a flow rate of 50 ml/min, and the animal was allowed to stay in the chamber for 10 min to adapt to the chamber environment before recording, followed by a recording of 20 minutes at room temperature. The signal was collected and analyzed with Clampfit software (Molecular Devices).
The variability of breathing frequency (f) was calculated from at least 200 successive breathing cycles as described previously (Zhang et al. 2011). Apnea (apneas / h) was counted only if a breathing cycle was longer than two prior breaths.
Brain Slice Preparation and Electrophysiology
Brain slices were prepared as described previously (Zhang, et al., 2010a). In brief, TH-ChR-Mecp2−/Y and control mice at 4-6 week-old were anesthetized as described above. The brainstem was obtained rapidly and placed in an ice-cold, sucrose-rich artificial cerebrospinal fluid (sucrose aCSF) containing (in mM) 200 sucrose, 3 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, 1. 25 NaH2PO4 and 10 D-glucose. The solution was bubbled with 95% O2 and 5% CO2 (pH 7. 40). Transverse pontine sections (300 μm) containing the LC area and transverse medullary slices (200 μm) containing the hypoglossal nucleus were obtained using a vibratome (1000 Plus, Vibratome, St. Louis, MO). The slices were transferred to normal aCSF in which the sucrose was substituted with 124 mM NaCl, allowed to recover at 33°C for 1 h, and then kept at room temperature before being used for recording.
One slice was transferred to a recording chamber that was perfused with oxygenated aCSF at a rate of 2 ml/min and maintained at 32–35°C. LC neurons were identified as described previously (Zhang, et al., 2010a). Whole-cell current clamp was performed in brain slices, and LC neurons were patched. Sutter pipette puller (Model P-97, Novato, CA) was used to pull the patch pipettes with a resistance of 3–5 MΩ. The pipette solution (in mM) contained 130 K-gluconate, 10 KCl, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP and 0.4 EGTA (pH 7. 3). The bath solution was normal aCSF bubbled with 95% O2 and 5% CO2 (pH 7. 40). In one set of experiments, α-adrenoceptors were blocked by adding phentolamine (10 μM, Sigma) to the bath solution. Recorded signals were amplified with an Axopatch 200B amplifier (Molecular Devices, Union City, CA), digitized at 10 kHz, filtered at 1 kHz, and collected with the Clampex software. The temperature was maintained at 33°C during recording by a dual automatic temperature control (Warner Instruments).
Optical Stimulation
A light source with high-speed switcher (Lambda GD-4, Sutter Instruments) was connected to the incident-light illuminator port of the microscope, and delivered blue light through a 470 nm bandpass filter. Light pulses were triggered by the Digitimer D4030. This light source provides ~20 mW/mm2, which was evaluated with a MRD500 photodiode (Motorola). The 10-20 ms light pulse was given to brain slices, which has been shown to produce maximal activation of neurons in our previous studies (Jin et al. 2016; Jin et al. 2013a).
Statistics analysis
The electrophysiological and plethysmograph data were analyzed with Clampfit 10.3 software (RRID:SCR_011323). Two-tailed Student’s t-test and Mann–Whitney U test were used to perform the statistical analysis. Delayed excitation (DE) was described as the time delay of the first action potential at the end of each hyperpolarization command, which was fit with a Boltzmann equation as normalized D = 1 /{1 + exp [− (V − V½) / k]} where D is the delay period, V is the hyperpolarizing membrane potential, V½ is the half-inactivation, and k is the slope factor. The difference was considered significant when P<0.05. Data are presented as means ± SEM.
Results
1. Generation of double transgenic mice
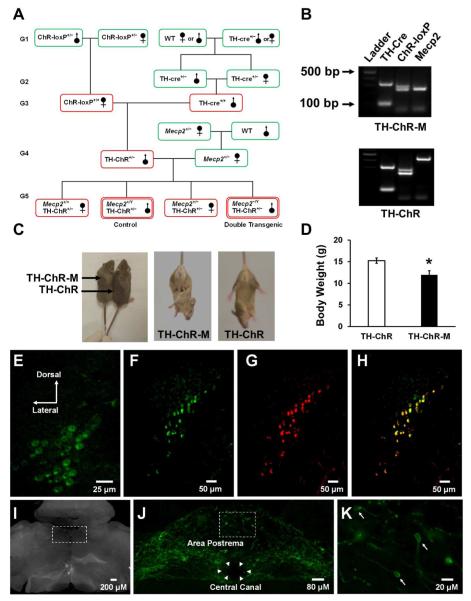
We took advantage of two strains of commercially available mice (TH-cre and ChR-loxP) and generated transgenic mice with ChR expression in catecholaminergic neurons. The heterozygous TH-ChR mice obtained were then used to cross-breed with heterozygous female Mecp2+/− mice. A half of their female offspring carrying ChR−/− was excluded for further studies. In the other half, mice with Mecp2+/Y and Mecp2+/− genotypes as well as ChR+/− were identified (Fig. 1A,B). The male TH-ChR-Mecp2−/Y and TH-ChR-Mecp2+/Y or TH-ChR mice were chosen as the experimental and control groups in the present study. In addition, a TH-ChR female was used as control as well.
Fig. 1. Generation of double transgenic RTT mice model, TH-ChR-Mecp2−/Y (TH-ChR-M).
A. Breeding paradigm to generate double transgenic mice. Green, mouse strains available commercially; red, mice generated be cross-breeding. B. Genotyping of transgenic TH-ChR-Mecp2−/Y and TH-ChR mice. TH-Cre expression: internal positive control = 324 bp, transgene = ~100 bp (this assay does not distinguish heterozygous from homozygous transgenic animals); ChR-loxP expression: mutant = 253 bp, heterozygote = 253 and 297 bp, wild type = 297 bp; Mecp2 expression: mutant = 240 bp, wild type = 465 bp. C. Photographs of TH-ChR-M and TH-ChR mice at five weeks old. RTT-like phenotypes were observed in the double transgenic mice, including hind limb clasping and low body weight. D. Significant lower body weight was found in TH-ChR-M mice (n=11 animals) when compared with littermate TH-ChR mice (n=11 animals) during postnatal week four to nine. Data are presented as mean ± SEM. E. YFP fluorescence in LC neurons obtained from a double transgenic TH-ChR-M mouse. F-H. Most DBH-immunoreactive LC neurons showed YFP in a TH-ChR mouse. LC neurons were detected with anti-GFP antibodies (F) and anti-DBH antibodies (G). The overlay of F and G indicates most LC neurons express YFP (H). I, Non-fluorescent images of sections in J and K. J,K. In the medulla oblongata, catecholaminergic neurons were labeled with anti-GFP antibodies in area postrema. Arrow heads, the central canal; arrows, cell bodies of catecholaminergic neurons. Note that J is obtained from the boxed area in I, and K is acquired from the boxed area in J.
In comparison to the littermate control TH-ChR mice 21.6 ± 1.4 g during age four to nine weeks old, the TH-ChR-Mecp2−/Y mice showed lower body weight 15.1 ± 0.9 g (P = 0.018 unpaired t-test). All double transgenic mice also exhibited hindlimb clasping, the characteristic features found in several mouse models of RTT at five weeks. The Fig. 1C was taken at week five. (Fig. 1C,D).
2. Morphological Evidence for YFP expression in LC neurons
YFP fluorescence was observed in LC neurons in brain slices obtained from either TH-ChR mice or TH-ChR-Mecp2−/Y mice (Fig. 1E). Immunocytochemical studies showed that nearly all YFP-positive neurons were also immunoreactive to DBH antibodies (Fig. 1F-G). YFP expression was clearly seen in other brainstem areas including area postrema (Fig. 1J,K), which can be identified in dark filed images (Fig. 1I).
3. Breathing Abnormality (f variation, apnea count)
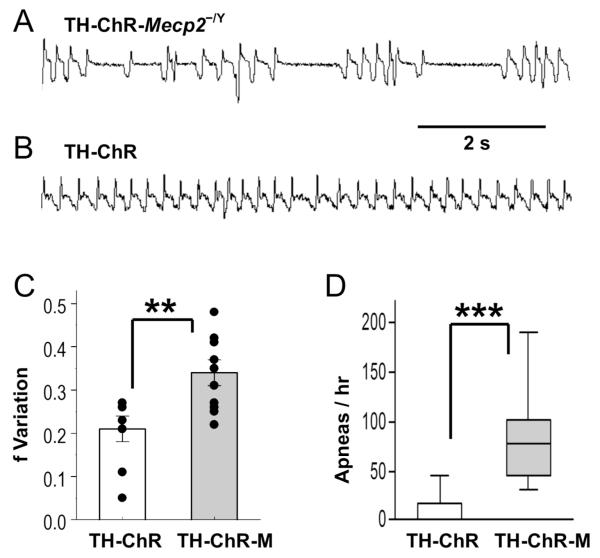
Several RTT-like breathing abnormalities have been found in Mecp2-null mice, including high apnea rate and breathing frequency (f) variation (Viemari, et al., 2005, Zhang, et al., 2011). Both of these breathing abnormalities were observed in double transgenic TH-ChR-Mecp2−/Y mice showing alternating periods of fast and slow respiratory frequency and a high rate of apnea (Fig. 2A). Such breathing abnormalities were not seen in TH-ChR mice (Fig. 2B). Statistical analysis of breathing f variation in controls 0.21 ± 0.03 vs. double transgenic mice 0.34 ± 0.03 (P = 0.003 unpaired t-test) and apnea counts 0 vs. double transgenic mice 75.5 (P<0.001, U9,10 = 2.5, Mann-Whitney U test) in the same age mice indicated that both RTT-like symptoms were significantly higher in the TH-ChR-Mecp2−/Y mice than in the TH-ChR (Fig. 2C,D).
Fig. 2. Breathing activity of TH-ChR-Mecp2−/Y (TH-ChR-M) and TH-ChR mice measured in plethysmography.
A,B. Real-time trace of breathing activity of a double transgenic (upper) and control mice (lower). Multiple apneas can be identified in double transgenic mice but not in control mice. C,D. Statistically, the TH-ChR-M (n=10 animals) exhibited significantly larger breathing frequency (f) variability and more apnea counts than the control mice (n=9 animals). **, P<0.01, ***, P<0.001 (Mann-Whitney U test for apnea count and Student’s t-test for f variation). Data are presented as mean ± SEM.
4. Electrophysiological Recording from LC neurons in TH-ChR-Mecp2−/Y mice
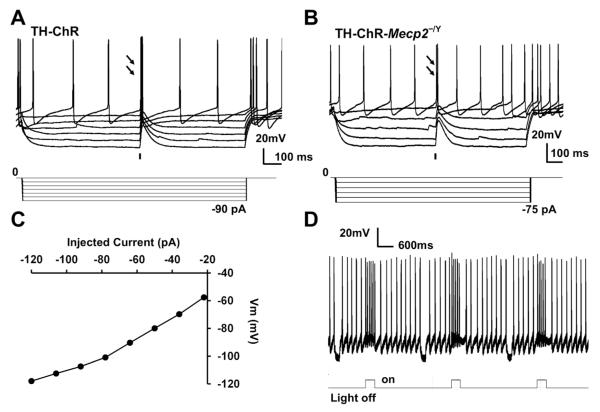
To confirm the functional expression of ChR in LC neurons, we patched these cells and exposed them to blue light (470nm) in brain slices in vitro. In whole-cell recording, these cells from the TH-ChR and TH-ChR-Mecp2−/Y mice displayed depolarization in response to a 10 ms optostimulation (Fig. 3A,B), and strong inward rectification (Fig. 3C). The depolarization amplitude increased with hyperpolarizing pulses and decreased with depolarizing pulses indicating that it was produced by non-selective cationic channels. Optostimulation also raised firing activity in spontaneously firing LC neurons, consistent with the ChR expression in the cell (Fig. 3D).
Fig. 3. Optostimulation of LC neurons.
A. Light-evoked depolarization in an LC neuron from a TH-ChR mouse by 10 ms blue light pulses (470 nm) as indicated by the black bar. The light-evoked depolarization is larger with hyperpolarization. Arrows indicate action potentials evoked by optostimulation. B. Light-evoked depolarization an LC neuron from a TH-ChR-Mecp2−/Y mouse by 10 ms blue light pulses (470 nm) as indicated by the black bar. Arrows indicate action potentials evoked by optostimulation (n=3 cells/1 mouse). C. I-V plot of a LC neuron from a TH-ChR-Mecp2−/Y mouse. D. Optostimulation enhanced firing activity of an LC neuron with high fidelity from a TH-ChR-Mecp2−/Y mouse.
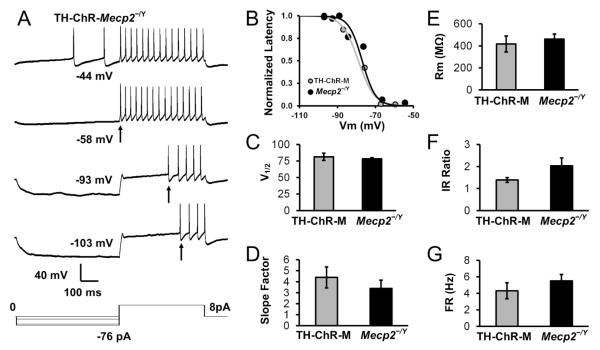
The LC cells from TH-ChR-Mecp2−/Y mice exhibited typical membrane properties of LC neurons in Mecp2-null mice. Delayed excitation (DE) was seen, which as one of most characteristic properties of LC neurons occurs as delayed action potentials following hyperpolarization (Fig. 4A). LC neurons in the TH-ChR-Mecp2−/Y mice had a V½ of 81.4 ± 5.4 mV (n=4 cells/2 mice), which was not significantly different from Mecp2-null mice, 78.4 ± 1.8 mV (n=5 cells/3 mice) (P = 0.585 unpaired t-test, Fig. 4B,C). The slope factor of double transgenic mice is 4.4 ± 1.0, which had no significant difference compared to Mecp2-null mice at 3.4 ± 0.8 (P = 0.43, unpaired t-test, Fig. 4D). The input resistance of TH-ChR-Mecp2−/Y mice was 416.5 ± 46.3 MΩ (n=4 cells/2 mice) and 461.3 ± 46.18 MZ (n=5 cells/3 mice) in Mecp2-null mice, which were not significantly different (P = 0.606 unpaired t-test, Fig. 4E). The Inward Rectification ratio of double transgenic mice 1.4 ± 0.1 was not significantly different from Mecp2-null mice 2.0 ± 0.4 (P = 0.166, unpaired t-test, Fig. 4F). The spontaneous firing frequency of TH-ChR-Mecp2−/Y mice was 4.3 ± 1.0 Hz (n=4 cells/2 mice) and 5.5 ± 0.8 Hz (n=5 cells/3 mice) (P = 0.361, unpaired t-test, Fig. 4G). The average resting membrane potential of TH-ChR-Mecp2−/Y mice was −43.7 ± 1. mV (n=4 cells/2 mice) compared to −41.9 ± 1.0 mV in Mecp2-null mice (n=5 cells/3 mouse) (P = 0.321, unpaired t-test, data not shown). Thus, our results indicated that the introduction of the exogenous ChR protein did not alter the membrane properties of LC cells in double transgenic mice.
Fig. 4. Electrophysiological properties of LC neurons.
A. Whole cell current clamp recording from an LC neuron TH-ChR-Mecp2−/Y (TH-ChR-M) mice. The LC neuron showed delayed excitation (DE) with a series of hyperpolarizing steps followed by a depolarizing pulse. Step increases in hyperpolarization elongated the delay of the first spike (indicated by arrows). B. The relationship between action potential delays and the conditioning membrane potentials was fitted with the Boltzmann equations in TH-ChR-M and Mecp2-null mice and plotted. C-D. There was no difference in the V½ and slope factor between TH-ChR-M mice (n=4 cells/2 mice) and Mecp2-null (n=5 cells/3 mice). E-G. There was no significant difference in input resistance (P = 0.606 unpaired t-test), inward rectification (P = 0.166, unpaired t-test), and firing rate (P = 0.361, unpaired t-test) between TH-ChR-M mice (n=4 cells/2 mice) and Mecp2-null (n=5 cells/3 mice).
5. Modulation of brain stem neurons by optostimulation of NE-ergic neurons
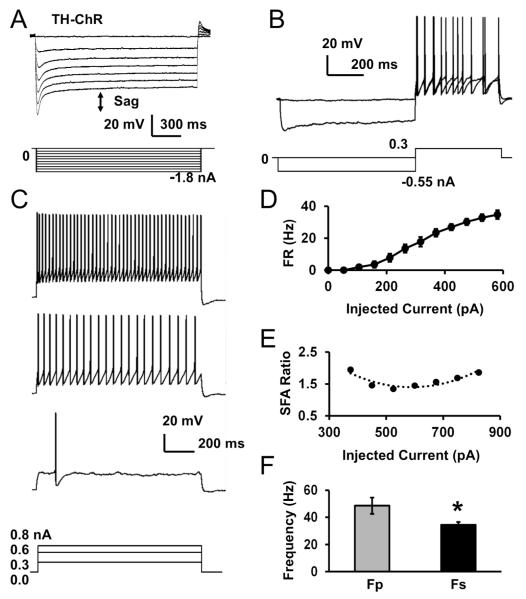
In our previous study, we have shown the postsynaptic modulation of hypoglossal motoneurons (HNs) by norepinephrine (Jin et al. 2013b). These neurons may remain to be modulated in the medullary slice preparation as the NE-ergic neurons are found in area postrema (Viemari, et al., 2005). To determine how optostimulation of NE-ergic cells expressing ChR modulates HNs, we recorded HNs while giving optostimulation to NE-ergic terminals. The HNs in TH-ChR mice (n=9 cells/2 mice) showed typical properties as shown in previous studies such as resting membrane potential −58.1 ± 1.2 mV, input resistance 56.4 ± 11.0 MΩ (n=9 cells/2 mice), whole-cell capacitance 63.1 ± 8.3 pF, and display of a sag potential in response to hyperpolarizing currents with an average of 18.3 ± 3.3 mV which was followed by post-inhibitory rebound (Fig. 5A). The sag was defined as the difference between the peak voltage during the current injection and the steady-state voltage. HNs did not show delayed excitation (Fig. 5B) as LC neurons, while displaying increased firing activity by each depolarizing pulse. The cells showed moderate spike frequency adaptation (SFA), which is described as Fp/Fs ratio (Fig. 5C,D,E). In response to the same depolarizing pulse, the firing rate of the first two action potentials at the peak state (Fp) 48.5 ± 6.0 Hz was significantly higher than that at steady state (Fs) 34.4 ± 2.2 Hz (P = 0.043, paired t-test, Fig. 5F)
Fig. 5. Electrophysiological properties of hypoglossal neurons (HNs) from TH-ChR mice.
A. An HN was recorded in current clamp. The cell showed typical sag potential described as the voltage difference between the peak and steady-state voltages in response to steps of hyperpolarizing pulses (indicated with the arrow). The cell also displayed a post-inhibitory rebound of depolarization, which is the difference between the baseline and rebound voltage, after termination of hyperpolarizing pulses. B. The HN did not show DE. C. Increased firing activity and SFA was seen in the cell with depolarization currents. D. The average firing rate was calculated and graphed with depolarizing current injections, n=9 cells/2 mice. E. A moderate SFA ratio was seen in the HN. F. Fp and Fs were compared, n=9 cells/2 mice. Data are presented as mean ± SEM (*, P<0.05; Student’s t-test).
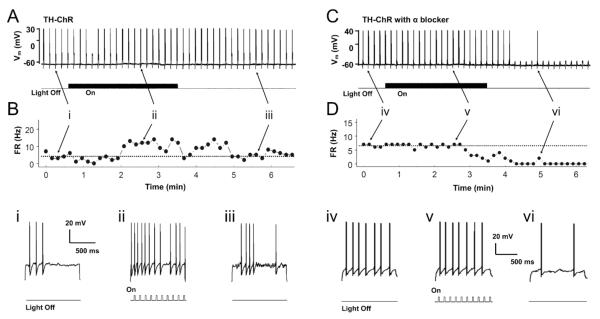
HNs were typically silent at rest in brain slices except for one cell. Therefore, we measured evoked firing activity of HNs by giving a depolarizing pulse at a level slightly above the firing threshold. Firing activity of HNs was measured at baseline, during light stimulation, and without light stimulation as washout. Light stimulation consisting of 20ms blue light pulses at 10 Hz increased the evoked firing activity, which returned to baseline levels during washout when the light was off (Fig. 6A, B). On average, the firing activity of these HNs in control TH-ChR mice increased from baseline 8.4 ± 1.9 Hz (n=9 cells/2 mice) to 12.3 ± 1.5 Hz during light stimulation, which was significantly increased (P = 0.003, paired t-test, Fig. 7C). Although optostimulation augmented HN firing activity, the light did not produce any visible depolarization.
Fig. 6. The response of hypoglossal neurons to NE-dependent optostimulation in TH-ChR mice.
A. Evoked firing activity of an HN was measured during baseline, optostimulation and light-off recovery. B. Measurement of instantaneous firing rate (FR) of the HN. C,D. Firing activity of another HN recorded in the presence of α-adrenoceptors blocker phentolamine (10 μM). Recording traces in i through vi were obtained from their corresponding sites above with expansion.
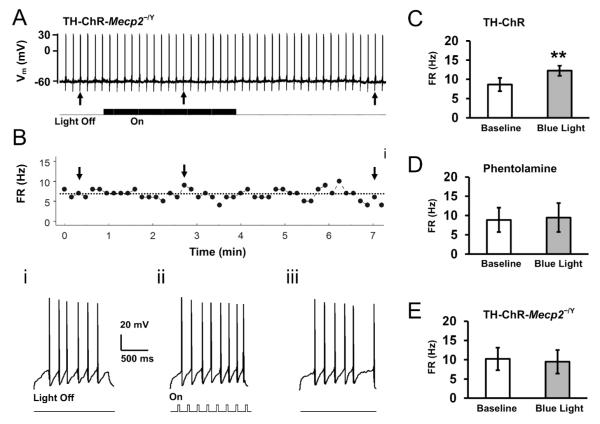
Fig. 7. The NE-dependent optostimulation has no effect on hypoglossal neurons in TH-ChR-Mecp2−/Y mice.
A. Evoked firing activity of an HN from a TH-ChR-Mecp2−/Y mouse. B. The instantaneous firing rate of the HN. Recording traces in i through iii were obtained from their corresponding sites above with expansion. C. Optical NE-ergic stimulation augmented firing activity of HNs in TH-ChR mice, n=9 cells/2 mice. Data are presented as mean ± SE (**, P<0.01; Student’s t-test). D. The same optical NE-ergic stimulation failed to enhance firing activity in TH-ChR mice when phentolamine (10 μM) was added to the perfusion solution (P>0.05, n=4 cells/1 mouse). E. The NE-dependent optostimulation had no significant effect on firing in TH-ChR-Mecp2−/Y mice (P>0.05, n=6 cells/2 mice).
In our previous study, we have also shown that NE modulates HNs through postsynaptic α-adrenoceptors. To determine if the effects of light stimulation were mediated through α-adrenoceptors, HNs from control TH-ChR mice were measured during light stimulation in the presence of the α-adrenoceptor antagonist phentolamine (10 μM). Light stimulation failed to increase firing activity in the presence of phentolamine. The evoked firing rate was 9.1 ± 3.3 Hz at baseline vs. 9.1 ± 3.5 Hz (n=4 cells/1 mouse) during optostimulation (Fig. 6C,D, Fig. 7D, P = 0.985 paired t-test).
6. Modulation of brain stem neurons by optostimulation of NE-ergic neurons in TH-ChR-Mecp2−/Y Mice
The Mecp2 disruption in mice causes reductions in NE content (Roux et al. 2008). To show how the NE-ergic synaptic terminals respond to optostimulation, we measured evoked firing activity of HNs before, during and after light stimulation of presynaptic NE-ergic terminals as mentioned above. The evoked firing rate of HNs from double transgenic mice was 10.2 ± 2.9 Hz (n=6 cells/2 mice) at baseline, which was not significantly different from controls (n=9 cells/2 mice, P = 0.908, unpaired t-test). Optostimulation augmented the firing rate of the HNs in double transgenic mice modestly to 9.5 ± 3.7 Hz (Fig. 7A,B), which was not significantly different from the baseline level (P = 0.595, paired t-test, Fig. 7E). In comparison to the TH-ChR neurons (Fig. 7E), the average response of firing rate to light stimulation was significantly lower in TH-ChR-Mecp2−/Y mice (−0.7 ± 1.6 Hz, n=6 cells/2 mice) than in control mice (3.8 ± 0.9 Hz, n=9 cells/2 mice; P = 0.01, unpaired t-test).
Discussion
We have shown the generation of a new double transgenic RTT mouse model with ChR-eYFP expression in catecholaminergic neurons in this study. TH is a rate-limiting enzyme for catecholamine synthesis. The TH promoter has been previously used to direct α-synuclein expression in dopaminergic neurons to generate Parkinson’s disease model (Daher et al. 2009; Richfield et al. 2002; Tofaris et al. 2006) and GFP expression in NE-ergic neurons (Lammel et al. 2015; Wang et al. 2014). Consistently, our results indicate that ChR-eYFP is expressed in LC neurons showing YFP fluorescence and functional ChR activity in our TH-ChR mice.
The mouse strain with the loxP ChR-eYFP was used in this study. The loxP ChR-eYFP cassette was constructed in the ROSA26 locus known to be constitutively active, which is consistent with our finding that the heterozygous mice express ChR-eYFP. This as well as the fact that the ROSA26 locus is located on chromosome 6, and the Mecp2 is on the X chromosome favors the generation of double transgenic mice. Indeed, we have successfully obtained the TH-ChR-Mecp2−/Y double transgenic mice with extensive mouse breeding and step-by-step genotyping. The reasons for using the male mouse model are that 1) the Mecp2−/Y males offer a completely Mecp2-null condition that is not always available in Mecp2+/− females owing to uncontrolled X-chromosome inactivation; 2) the male Mecp2−/Y mice have been widely used as a model of RTT, and the results obtained from this model can be easily compared and evaluated with existing literature.
The TH-ChR-Mecp2−/Y mice exhibit typical phenotypes seen in other mouse models of RTT, including hypoactivity, low body weight, short life span, and hind limb clasping. They also show clear breathing disorders, including significant higher apnea counts and more frequent breathing f variation than the TH-ChR control. Also similar to the existing mouse models of RTT are the high infant fatality rate and short lifespan (not shown). Nevertheless, our breeding strategy allows production of the TH-ChR-Mecp2−/Y mice roughly in the same successful rate as other mouse models of RTT that we have used.
The chance to get male double transgenic mice based on our breeding scheme is 12.5%. We realized that the availability of the double transgenic mice limited the power analysis. It took three to four months to generate F1 generation of double transgenic mice and the average litter size was approximately 6 with 12.5% mutation ratio. This ratio can be enhanced to ~25% by crossbreeding male TH-ChR and female TH-ChR-Mecp2−/+ mice. The production rate and sustainable generation of double transgenic mice suggest that these mice may provide another way to study RTT by targeting on specific neurons in central nervous system or certain tissues in peripheral system as well.
Immunohistochemistry identification of catecholaminergic neurons with anti-YFP antibodies in both pons and medulla were clearly seen while these neurons are exhibiting endogenous genetically engineered fluorescence (Fig. 2). Further evaluation of ChR expression indicates that ChR is fully functional in LC neurons in double transgenic mice, producing depolarization and firing activity in the cells and NE-ergic modulation with blue light stimulation. The knock-in of ChR in LC neurons did not alter intrinsic properties of LC neurons as well. Therefore, this new strain of double transgenic mice, TH-ChR-Mecp2−/Y, seems to allow access to LC neurons with high specificity as well as excellent temporal and spatial resolutions.
One group of brainstem neurons targeted by NE modulation is the HNs. NE augments their firing activity by pre- and post-synaptic mechanisms (Jin, et al., 2013). Such an NE-ergic modulation is defective in Mecp2-null mice, suggesting that HNs are ideal for testing NE modulation in our TH-ChR-Mecp2−/Y mice. However, the hypoglossal nucleus is located remotely from the LC, making the joined brain slice studies of these two types of neurons very difficult, if possible. Because of the existence of local NE-ergic neurons in the close vicinity of the hypoglossal nucleus and because of the possibility of optostimulation of axonal terminals of NE-ergic neurons, we have tested the NE-ergic modulation of HNs by optostimulation of NE release in this study. In TH-ChR mice, the HNs firing activity is clearly augmented with optostimulation. Such an effect was not seen in the presence of the non-selective α-adrenoceptors antagonist phentolamine, indicating that HNs are modulated by NE through α-adrenoceptors. Notably, the HNs became silent during washout, which seems due to residual NE modulation that is suppressed by phentolamine.
In TH-ChR-Mecp2−/Y mice, we have found that the augmentation of HN firing activity by optostimulation of NE-ergic neurons is drastically impaired. Indeed, optostimulation did not show any significant effect on the HN firing activity. Previous studies indicate that the Mecp2 disruption causes defects in NE biosynthesis (Viemari et al. 2005). Thus, the impaired NE-ergic modulation of HNs is likely to be caused by a decrease in NE content at synaptic terminals, a reduction in NE release from NE-ergic synapses, or both. In the presence of these defects, optoexcitation of NE-ergic neurons and axonal terminals apparently does not help to improve the NE release. Because the strong optostimulation used in the present study fails to augment NE-ergic modulation, an increase in spontaneous firing of the NE-ergic neurons may not benefit much to the NE release either. Instead, the excessive neuronal excitability might even worsen the NE-ergic modulation by perhaps depleting NE in presynaptic terminals. Therefore, LC neuronal hyperexcitability appears harmful to the NE-ergic modulation under the condition of Mecp2 disruption.
Significance Statement.
RTT is a neurodevelopmental disease that occurs in 1 in 10,000 live-birth females globally. Over 25% of RTT patients die of unexplained causes, which is mostly attributable to their respiratory disorders and autonomic dysfunction involving the NE-ergic system in the brainstem. Thus, understanding the cellular mechanism for the abnormal NE-ergic system in RTT models is clinically significant. One characteristic feature of the NE-ergic defects is hyperexcitability in LC neurons, while the effect of the hyperexcitability on NE release is unclear. Here we created a double transgenic mouse model that allowed accesses to LC neuronal excitability with light. Our results suggest that the Mecp2 disruption appears to impair NE release from axonal terminals, which cannot be compensated with extensive neuronal excitation.
Abbreviations
- Mecp2
Methyl-CpG-binding protein 2 gene
- RTT
Rett syndrome
- TH
tyrosine hydroxylase
- DBH
Dopamine beta-hydroxylase
- ChR
channelrhodopsin
- LC
locus coeruleus
- NE
norepinephrine
- HNs
hypoglossal neurons
- SFA
spike frequency adaptation
- DE
delayed excitation
- IR
inward rectification
Footnotes
This work was supported by the NIH (NS073875). SZ is a Molecular Basis of Disease (MBD) fellow of Georgia State University.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: SZ, CMJ, CJ. Acquisition of data: SZ, CMJ, NC, HX, WZ, WY. Analysis and interpretation of data: SZ, CMJ, CJ. Drafting of the manuscript and Critical revision of the article: SZ, CMJ, CJ. Statistical analysis: SZ, CMJ, CJ. Obtained funding: CJ.
References
- Daher JP, Ying M, Banerjee R, McDonald RS, Hahn MD, Yang L, Flint Beal M, Thomas B, Dawson VL, Dawson TM, Moore DJ. Conditional transgenic mice expressing C-terminally truncated human alpha-synuclein (alphaSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Molecular neurodegeneration. 2009;4:34. doi: 10.1186/1750-1326-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature genetics. 2001;27(3):322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Ide S, Itoh M, Goto Y. Defect in normal developmental increase of the brain biogenic amine concentrations in the mecp2-null mouse. Neuroscience letters. 2005;386(1):14–17. doi: 10.1016/j.neulet.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Jin X, Li S, Bondy B, Zhong W, Oginsky MF, Wu Y, Johnson CM, Zhang S, Cui N, Jiang C. Identification of a Group of GABAergic Neurons in the Dorsomedial Area of the Locus Coeruleus. PloS one. 2016;11(1):e0146470. doi: 10.1371/journal.pone.0146470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Zhong W, Jiang C. Time-dependent modulation of GABA(A)-ergic synaptic transmission by allopregnanolone in locus coeruleus neurons of Mecp2-null mice. American journal of physiology Cell physiology. 2013a;305(11):C1151–1160. doi: 10.1152/ajpcell.00195.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XT, Cui N, Zhong W, Jin X, Wu Z, Jiang C. Pre- and postsynaptic modulations of hypoglossal motoneurons by alpha-adrenoceptor activation in wild-type and Mecp2(−/Y) mice. American journal of physiology Cell physiology. 2013b;305(10):C1080–1090. doi: 10.1152/ajpcell.00109.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julu PO, Kerr AM, Apartopoulos F, Al-Rawas S, Engerstrom IW, Engerstrom L, Jamal GA, Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Archives of disease in childhood. 2001;85(1):29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L, Malenka RC. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85(2):429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40(2):67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsaki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature neuroscience. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Experimental neurology. 2002;175(1):35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Roux JC, Dura E, Moncla A, Mancini J, Villard L. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. The European journal of neuroscience. 2007;25(7):1915–1922. doi: 10.1111/j.1460-9568.2007.05466.x. [DOI] [PubMed] [Google Scholar]
- Roux JC, Dura E, Villard L. Tyrosine hydroxylase deficit in the chemoafferent and the sympathoadrenergic pathways of the Mecp2 deficient mouse. Neuroscience letters. 2008;447(1):82–86. doi: 10.1016/j.neulet.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O'Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(15):3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(50):11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard L, Roux JC. [Noradrenaline deficiency as the origin of respiratory disorders in Rett syndrome an animal model] Medecine sciences : M/S. 2006;22(1):81–83. doi: 10.1051/medsci/200622181. [DOI] [PubMed] [Google Scholar]
- Wang X, Pinol RA, Byrne P, Mendelowitz D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem alpha1 and beta1 receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(18):6182–6189. doi: 10.1523/JNEUROSCI.5093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui N, Wu Z, Su J, Tadepalli JS, Sekizar S, Jiang C. Intrinsic membrane properties of locus coeruleus neurons in Mecp2-null mice. American journal of physiology Cell physiology. 2010;298(3):C635–646. doi: 10.1152/ajpcell.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Su J, Cui N, Gai H, Wu Z, Jiang C. The disruption of central CO2 chemosensitivity in a mouse model of Rett syndrome. American journal of physiology Cell physiology. 2011;301(3):C729–738. doi: 10.1152/ajpcell.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Cui N, Jin X, Oginsky MF, Wu Y, Zhang S, Bondy B, Johnson CM, Jiang C. Methyl CpG Binding Protein 2 Gene Disruption Augments Tonic Currents of gamma-Aminobutyric Acid Receptors in Locus Coeruleus Neurons: IMPACT ON NEURONAL EXCITABILITY AND BREATHING. The Journal of biological chemistry. 2015;290(30):18400–18411. doi: 10.1074/jbc.M115.650465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, Milstien S, Butler IJ, Smith EO, Kaufman S, Glaze DG, Percy AK. Cerebrospinal fluid biogenic amines and biopterin in Rett syndrome. Annals of neurology. 1989;25(1):56–60. doi: 10.1002/ana.410250109. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Percy AK, Glaze DG, Butler IJ, Riccardi VM. Reduction of biogenic amine levels in the Rett syndrome. The New England journal of medicine. 1985;313(15):921–924. doi: 10.1056/NEJM198510103131504. [DOI] [PubMed] [Google Scholar]