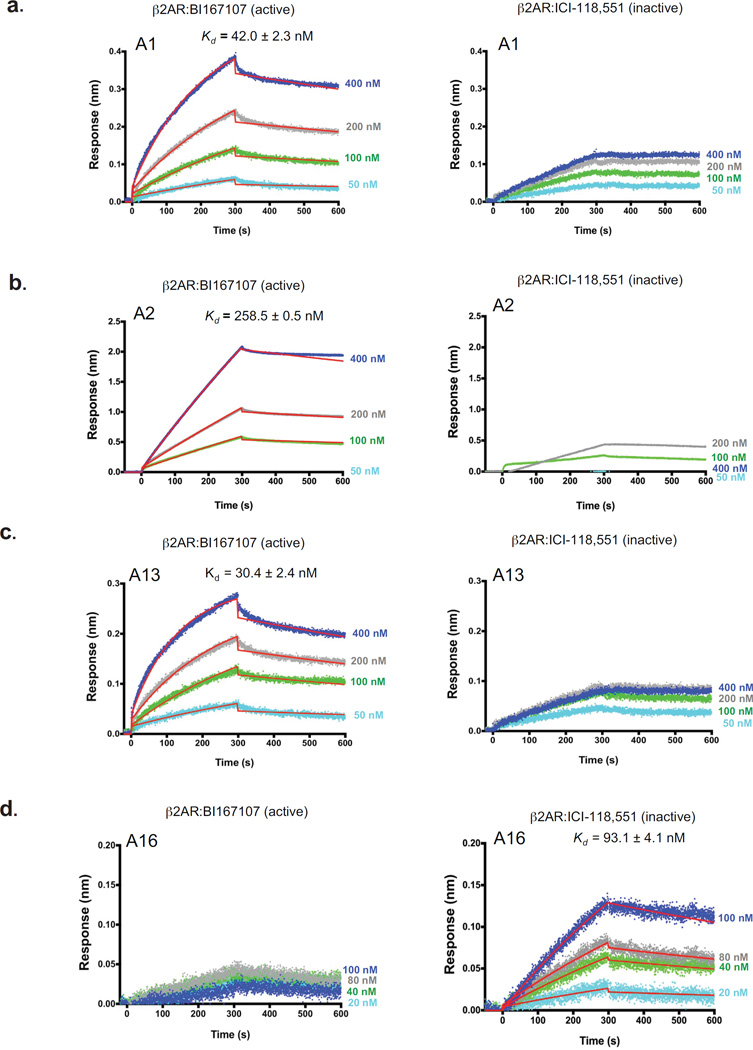
Figure 2. Aptamers distinguish between inactive and active conformations of the β2AR.
Binding kinetic profiles for the interactions of four biotinylated aptamers with BI167107-bound (active) β2AR or ICI-118,551-bound (inactive) β2AR as analyzed using biolayer interferometry (BLI). (a–d) Representative sensorgrams for the interactions of four biotinylated aptamers with BI167107-bound β2AR (left panels) or ICI-118,551-bound β2AR (right panels): A1 (a), A2 (b), A13 (c), and A16 (d). Data was globally fit to 1:1 binding model as described in methods. Kd (dissociation constant) is shown as the ratio of koff (dissociation) to kon (association) rate constants. Each Kd value represents the mean affinity values ± s.e.m. of three independent experiments. Blue, gray, green, and light blue curves represent the measured responses for each tested concentration of β2AR (BI167107 or ICI-118,551-bound β2AR). Whereas overlay the curves in red show the global fitting results of the binding data.