Abstract
Background
Hypertension is a common complication and is an important risk factor for graft loss and adverse cardiovascular outcomes in pediatric kidney transplantation. Ambulatory blood pressure monitoring (ABPM) is the preferred method to characterize blood pressure status.
Methods and Materials
We conducted a retrospective review of a large cohort of children and young adults with kidney transplant to estimate the prevalence of abnormal ambulatory blood pressure (ABP), assess factors associated with abnormal ABP, and examine whether ambulatory hypertension is associated with worse allograft function and left ventricular hypertrophy (LVH).
Results
Two hundred and twenty-one patients had ABPM and 142 patients had echocardiographic results available for analysis. One third of the patients had masked hypertension, 32% had LVH, and 38% had estimated GFR<60ml/min/1.73m2. African-American race/Hispanic ethnicity and requirement for more than one antihypertensive medication were independently associated with having masked hypertension. In a multivariate analysis, abnormal blood pressure (masked or sustained hypertension combined) was an independent predictor for LVH among patients not receiving antihypertensive treatment (p=0.025). In a separate analysis, the use of antihypertensive medications was independently associated with worse allograft function (p=0.002), although abnormal blood pressure was not a significant predictor.
Conclusion
In young kidney transplant recipients, elevated ABP is frequently unrecognized and undertreated. The high prevalence of abnormal ABP, including masked hypertension, and its association with LVH supports the case for routine ABPM and cardiac structure evaluation as the standard of care in these patients.
INTRODUCTION
Hypertension is a known common complication after pediatric kidney transplantation1,2,3 and is associated with worse short and long term graft function and with left ventricular hypertrophy (LVH)4-7. Over the last two decades, ambulatory blood pressure monitoring (ABPM) has been utilized more frequently to characterize blood pressure (BP) status in patients with chronic kidney disease (CKD) because of its better ability to stratify cardiovascular risk and predict progression of CKD8-12. Studies in pediatric kidney transplant recipients have confirmed high prevalence of abnormal ambulatory blood pressure (ABP), including masked hypertension, a condition characterized by normal BP in clinic but elevated BP outside the medical provider’s office13-22. Yet, these published studies are generally small, use different definitions of abnormal ABP, and most of them do not address association of masked or sustained hypertension with allograft function or CV outcomes.
We conducted a study evaluating ABP patterns in a large cohort of kidney transplant recipients from the Midwest Pediatric Nephrology Consortium (MWPNC) with the following aims: 1) estimate the prevalence of ambulatory hypertension and assess factors associated with abnormal ABP and 2) examine the association of ambulatory hypertension with LVH and allograft function. We hypothesized that ambulatory hypertension would be associated with LVH and worse allograft function.
MATERIALS AND METHODS
A retrospective analysis of children and young adults with kidney transplants who had ABPM between January 2010 and December 2014 was carried out at 6 centers (via collaboration through the MWPNC). The study was reviewed and approved by the Institutional Review Boards of all participating centers.
Inclusion criteria were a functioning kidney transplant and age <23 years at the time of ABPM. Charts were reviewed for demographic information (age, gender, race), etiology of end-stage renal disease (glomerular versus structural/congenital), medications, history of prior transplants, dialysis prior to transplant, prior episode of rejection), anthropometric parameters (height, weight), casual blood pressure, and laboratory data (serum creatinine and hemoglobin) at time of the first ABPM after transplantation. Medication information collected specifically included all immunosuppressive agents and antihypertensive agents. Allograft function was determined based on the bedside Schwartz formula for patients younger than 18 years23 and CKD-EPI formula for patients 18-23 years24.
Echocardiographic data including left ventricular mass were collected, with analysis restricted to subjects who received an echocardiogram within 6 months of ABPM. Left ventricular hypertrophy was defined as left ventricular mass index (LVMI) ≥95th percentile for age and gender25.
Spacelabs 90217 monitor (SpaceLabs Healthcare, Issaquah, WA) was used for all ABPM studies. All centers utilized standard methodology to measure BP: for wake hours - every 20 minutes; for sleep hours measurements were performed either every 20 or 30 min.
The ABP parameters of interest included mean systolic and diastolic BP for wake, sleep, and 24-hour periods. From this, systolic and diastolic BP dip status was determined by calculating percent nocturnal drop in mean BP from waking mean values. In addition, wake and sleep BP loads were calculated as the percent of readings at or above the 95th percentile, based on published normative data26. Ambulatory BP index was calculated as the mean ABP divided by the corresponding 95th percentile. Thus, an index of 1 indicates ABP equal to the threshold value for a clinical diagnosis of hypertension, and an index of 1.1 is 10% above that threshold27. Since the 95th percentile is gender and height specific, this measure allows for comparison of BP across a wide range of pediatric normal values.
In this study, we applied the recently published American Heart Association (AHA) revised classification of BP status in children according to casual blood pressure (CBP) and ABP measurements28. Normal: CBP <90th percentile and mean daytime and nighttime, systolic and diastolic ABP<95th percentile and BP load < 25%; White coat hypertension (WCH): CBP≥95th percentile, mean daytime and nighttime, systolic and diastolic ABP<95th percentile and BP load < 25%; Prehypertension: CBP ≥90th percentile or >120/80, mean daytime and nighttime, systolic and diastolic ABP<95th percentile, BP load (daytime or nighttime, systolic or diastolic) ≥ 25%; Masked hypertension: CBP <95th percentile, mean daytime or nighttime, systolic or diastolic ABP ≥ 95th percentile, BP load ≥ 25%; Ambulatory (sustained) hypertension: CBP ≥95th percentile, mean daytime or nighttime, systolic or diastolic ABP ≥ 95th percentile, BP load ≥ 25%.
BP status was then re-classified using a different method based on the 24h measurements (instead of day/and/or night separately), and using a BP load of >25% as the cutoff for hypertension. This method allows classification of all patients into four simplified BP categories (normal, WCH, masked, and sustained hypertension), and avoids several situations in which the BP status is unclassified according to the AHA classification. According to this classification, patients with CBP<95th percentile (normal/prehypertension) and BP load ≥25% were classified as having masked hypertension, those with casual prehypertension and normal ABP were classified as having normal BP, and those patients with casual hypertension and BP load ≥25% were classified as having sustained hypertension.
Statistical analysis
For descriptive statistics, categorical variables were reported as percentages, and continuous variables reported as median and interquartile ranges. For univariate analyses, demographic and clinical characteristics were compared between BP categories using chi-square testing for categorical variables and the Wilcoxon rank-sum test for continuous variables. Logistic regression was used to investigate the independent association of ABP status with LVH and decreased allograft function (eGFR < 60 ml/min/1.73m2). All variables associated with an eGFR < 60 ml/min/1.73m2 and LVH in univariate analyses (p < 0.15) were initially included in the model. Backward elimination was performed to determine variables included in the final model, with an inclusion criterion of p < 0.05. Odds ratios were reported for each independent predictor along with Wald 95% confidence intervals. All statistical analyses were performed using SAS 9.3 statistical software.
RESULTS
Cohort Characteristics
Two hundred and twenty one kidney transplant recipients had ABPM results available for analysis. Patient characteristics are summarized in Table 1. The majority were male and white, and received a living donor kidney. One third of the cohort were young adults. One hundred and fifty three patients were taking antihypertensive medications, and among them, 61% were taking one, 31% were taking two, and 7% were taking three BP medications; 67% were on calcium-channel blockers, 40% on angiotensin-converting-enzyme inhibitors (ACEI) or angiotensin-receptor-blockers (ARB), 25% on beta-blockers, and 5% were taking diuretics. Among 142 patients who had an echocardiogram within six months of their ABPM, 32% were found to have LVH. There was no significant difference in patient characteristics between those who had and who did not have echocardiography except higher hemoglobin level in the group without echocardiography (Supplemental Table). Thirty-eight percent of the cohort had CKD stage 3-4.
Table 1.
Patient Characteristics (n=221)
| Age, y*
Age ≥18, n (%) |
16.9 (13.9-19.4) 89 (39) |
| Male Gender, n (%) | 136 (62) |
| Race, n (%) White African-American/Biracial Hispanic Other/Unknown |
139 (63) 33 (15) 26 (12) 23 (10) |
| Age at Transplantation, y* | 12 (7.3-15.1) |
| Time post Transplantation, y* | 3.5 (1.3-7) |
| Primary Acquired Glomerular Disease, n (%) | 51 (23) |
| First Transplant, n (%) | 207 (94) |
| Living Donors, n (%) | 130 (59) |
| Prior Dialysis, n (%) | 145 (66) |
| History of Rejection, n (%) | 57 (26) |
| Immunosuppression, n (%) | |
| Calcineurin Inhibitors | 176 (80) |
| Steroids | 94 (43) |
| Mycophenolate Mofetil | 170 (79) |
| Overweight/Obese, n (%) | 78 (35) |
| Hemoglobin, g/dL* | 12.2 (11.1-13.4) |
| Anemia, n (%) | 47 (22) |
| Treated for hypertension, n (%) | 153 (68) |
| LVH§, n (%) | 46 (32) |
| eGFR, Schwartz/CKD-EPI (ml/min/1.73m2)* | 68 (54-89) |
| eGFR <60 ml/min/1.73m2, n (%) | 83 (38) |
Data are presented as median (IQR)
142 patients had echo data available for analysis
Blood Pressure Classification
Blood pressure status is summarized in Table 2. According to CBP, 49% had normal BP, 34% had pre-hypertension, and 17% had hypertension. As expected, abnormal BP was identified more frequently based on ABP measurements and, in large part related to nocturnal hypertension, reaching 22-26% hypertensive based on mean ABP and with 42-45% hypertensive based on BP load.
Table 2.
BP status (n=221)
| SBP | DBP | |
|---|---|---|
| Casual BP mmHg, median (IQR) BP index, median (IQR) BP index ≥ 1.0, n (%) |
||
| 117 (108-124) | 70 (62-77) | |
| 0.89 (0.83-0.95) 29 (13) |
0.82 (0.73-0.91) 16 (7) |
|
| 24-hour BP mmHg, median (IQR) BP index, median (IQR) BP index ≥ 1.0, n (%) Load (%), median (IQR) BP load >25%, n (%) |
||
| 118 (110-125) | 70 (66-74) | |
| 0.93 (0.86-0.98) | 0.89 (0.83-0.95) | |
| 42 (19) | 30 (14) | |
| 17 (4-38) 81 (37) |
15 (7-34) 76 (34) |
|
| Day BP mmHg, median (IQR) BP index, median (IQR) BP index ≥ 1.0, n, (%) Load (%), median (IQR) BP load >25%, n (%) |
||
| 121 (114-129) | 74 (69-79) | |
| 0.92 (0.86-0.97) 35 (16) |
0.88 (0.82-0.94) 20 (9) |
|
| 14 (3-35) | 11 (3-28) | |
| 76 (34) | 64 (29) | |
| Night BP mmHg, median (IQR) BP index, median (IQR) BP index ≥ 1.0, n (%) Load (%), median (IQR) BP load >25%, n (%) |
||
| 109 (102-117) | 62 (57-67) | |
| 0.93 (0.88-1) | 0.91 (0.85-0.98) | |
| 58 (26) | 49 (22) | |
| 16 (0-47) 92 (42) |
20 (7-40) 99 (45) |
|
| Dipping %, median (IQR) Abnormal (<10%) |
||
| 10 (6-14) 114 (52) |
15 (9-21) 61 (28) |
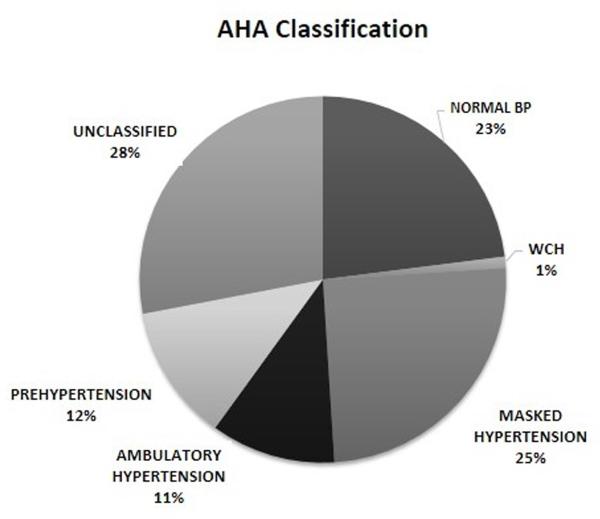
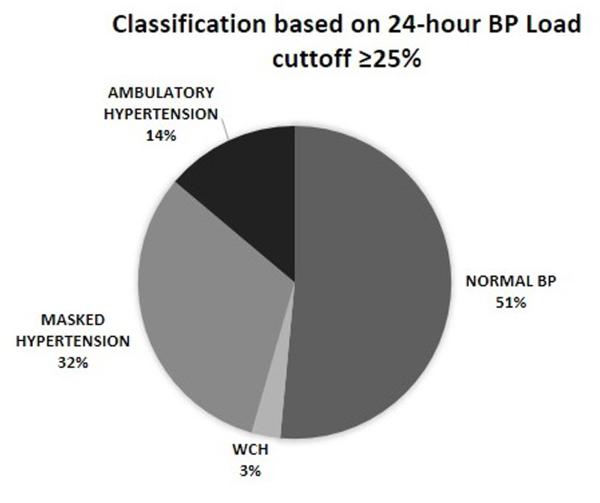
ABP patterns based on the combination of CBP and ABP are shown in Figure 1. According to the 2014 AHA classification (Figure 1A), 23% of the patients had normal BP. 12% had pre-hypertension, 25% had masked hypertension, and 11% had sustained hypertension. Only 1% of the patients were classified as having WCH. The largest group of patients (28%) was unclassified. This group was primarily composed of 1) patients with normal CBP, normal mean ABP but elevated BP load, and 2) patients with casual pre-hypertension but normal mean ABP results (mean BP <95th percentile and BP load <25%). Because many subjects were unclassified using the AHA classification, we re-classified the patients into four BP categories using 24-hour BP load ≥25% as the cutoff for hypertension (see methods). According to this classification (Figure 1B), 51% of patients had normal BP, 32% had masked hypertension, 14% had sustained hypertension, and 3% had WCH. Using this classification, 31 (84%) of 37 of patients with casual hypertension were confirmed to have sustained hypertension; among 76 patients with casual pre-hypertension, 40 (53%) had normal ABP and 47% had masked hypertension. All subsequent analyses were done using this classification.
Figure 1.
Ambulatory blood pressure patterns according to the 2014 AHA (A) classification and based on 24-hour blood pressure load cutoff to define hypertension (B) classification.
Factors associated with abnormal ABP
Patient characteristics according to their ABP/CBP status are summarized in Table 3. Both patients with masked and sustained hypertension were more likely to be either of African-American race or Hispanic ethnicity and were receiving more antihypertensive medications than normotensive patients. Patients with sustained hypertension were younger, received their transplant at a younger age, and were more likely to be on steroid treatment than those with either normal BP or masked hypertension.
Table 3.
Association of BP Status with Patient Characteristics*
| Normal blood pressure (n=114) |
Masked hypertension (n=70) |
Sustained hypertension (n=31) |
|
|---|---|---|---|
| Age**, years | 17.2 (14.4-20) | 17.4 (14.4-19.2) | 14.4 (10.3-16.5)a,b |
| Male Gender (%) | 64 (56) | 48 (69) | 19 (61) |
| African-American/Hispanic, n (%) | 20 (19) | 26 (40)a | 11 (44)a |
| Age at Transplantation**, years | 12.4 (8.4-16) | 12 (6.6-15.5) | 9 (5.7-12.6)a,b |
| Time post Transplantation**, years | 3.5 (1.1-7) | 3.9 (1.3-6.5) | 3 (1.4-9.6) |
| Acquired Glomerular Disease, n (%) | 27 (24) | 16 (23) | 8 (26) |
| Living Donors, n (%) | 69 (61) | 41 (59) | 16 (52) |
| Prior Dialysis, n (%) | 71 (63) | 48 (70) | 23 (74) |
| History of Rejection, n (%) | 30 (26) | 21 (30) | 9 (29) |
| Calcineurin Inhibitors, n (%) | 89 (80) | 58 (83) | 25 (86) |
| Steroids, n (%) | 45 (41) | 28 (40) | 18 (62)a,b |
| Overweight/Obese, n (%) | 36 (32) | 27 (39) | 14 (45) |
| Hemoglobin**, g/dL, | 12 (10.9-13) | 12.9 (11.6-13.6) | 11.7 (10.9-13.2) |
| Anemia, n (%) | 31 (27) | 11 (16) | 8 (26) |
| Treated for hypertension, n (%) | 74 (65) | 50 (71) | 25 (81) |
| ≥2 BP medications, n (%) | 22 (20) | 24 (34)a | 11 (35)a |
| LVMI (g/m2.7) § | 34 (30-40) | 37 (30-42) | 40 (35-48)a,b |
| LVH, n (%)§ | 21 (28) | 14 (33) | 11 (50)a |
| eGFR, Schwartz/CKD-EPI**
(ml/min/1.73m2) |
70 (55-92) | 69 (58-92) | 59 (45-78)a,b |
| eGFR <60 ml/min/1.73m2, n (%) | 43 (38) | 22 (31) | 16 (52) |
Characteristics of patients with WCH (n=6) are not presented due to the small number
Data are presented as median (IQR)
p<0.05 compared with normal BP
p<0.05 versus masked HTN
139 patients had echo results available for analysis
Blood pressure status and LVH
LVMI (median of 40 g/m2.7 vs. 34 g/m2.7, p=0.002) and the prevalence of LVH (50% vs. 28%, p=0.05) were higher in patients with sustained hypertension than in normotensive patients. However, median LVMI (37 g/m2.7) and the prevalence of LVH (33%) in patients with masked hypertension were similar to that observed in normotensive patients (Table 3).
Based on previous data suggesting patients with controlled hypertension (i.e., normotensive and receiving blood pressure medications) have a different cardiovascular risk profile than those without any history of hypertension17,20,29, BP status, LVMI and LVH were evaluated according the use of antihypertensive medications (Table 4). Among patients receiving antihypertensive medications, the distribution of patients amongst the BP categories was not significantly different than that observed in patients not receiving treatment. However the prevalence of LVH varied considerably between patients who were or were not on antihypertensive medications. Patients with normal BP who were not taking antihypertensive medications had the lowest LVMI (31g/m2.7) and prevalence of LVH (14%), while patients with normal BP values but who were taking antihypertensive medications (i.e. controlled hypertension) exhibited a median LVMI (37g/m2.7) and a prevalence of LVH (37%) similar to patients with uncontrolled (either masked and sustained) hypertension. The differences among normotensive patients (normal BP and controlled hypertension) were seen despite similar level of BP control (BP index and BP load) in these two groups (Table 4). Among patients with controlled hypertension, there was no significant difference in the prevalence of LVH according to class of BP medications (ACEI/ARB vs. others, p=0.68).
Table 4.
Blood Pressure Parameters, LVH and Graft Function According to BP Treatment and BP Status§
| Variable | Off BP meds (n=68) | On BP Meds (n=153) | ||||
|---|---|---|---|---|---|---|
| Normal BP n=40 (59%) |
Masked hypertension n=20 (29%) |
Sustained hypertension n=6 (9%) |
Controlled hypertension n=74 (48%) |
Masked hypertension n=50 (33%) |
Sustained hypertension n=25 (16%) |
|
| 24H SBP Index* | 0.86 (0.81-0.91) | 0.96 (0.93-0.99) | 0.98 (0.94-1) | 0.87 (0.94-0.91) | 0.98 (0.95-1.02) | 1.03 (0.98-1.1) |
| 24H SBP load*, (%) | 3 (0-12) | 30 (19-43) | 32 (16-50) | 4 (2-12) | 40 (27-55) | 56 (48-89) |
| 24 DBP Index* | 0.85 (0.79-0.88) | 0.97 (0.93-0.99) | 0.93 (0.91-0.95) | 0.85 (0.81-0.88) | 0.95 (0.9-1) | 0.99 (0.92-1.08) |
| 24H DBP load*, % | 7 (3-14) | 34 (21-47) | 35 (30-40) | 7 (4-13) | 31 (21-43) | 39 (27-73) |
| LVMI*, g/m2.7 | 31 (29-37) | 39 (36-47)b | 41 (39-43) | 37 (31-41)b | 34 (29-41) | 40 (34-50)b |
| LVH, n (%) | 4/29 (14) | 5/13 (38)a | 3/4 (75) | 17/46 (37)b | 9/29 (31) | 8/18 (44)b |
| eGFR, ml/min/1.73m2 | 78 (65-96) | 81 (68-96) | 51 (41-79)b | 61 (49-87)b | 67 (54-89)b | 62 (47-77)b |
| eGFR <60 ml/min/1.73m2, n (%) | 7 (18) | 1 (5) | 4 (67)b | 36 (49)b | 21 (42)b | 12 (48)b |
Data are presented as median (IQR)
Characteristics of patients with WCH (n=6) are not presented due to the small number
p=0.07 compared with patients with normal BP and off BP meds
p<0.05 compared with normal BP and off BP meds
Multivariate logistic regression analysis was performed to evaluate the independent association of BP status with LVH (Table 5). The interaction term Hypertension x BP medication was significant (p<0.05), confirming a difference in the relationship between hypertension and LVH in patients receiving and not receiving antihypertensive medication. Hypertension was independently associated with LVH among those not receiving BP treatment (OR 5.4; 95% CI 1.2 – 23.7, p=0.025). However, the OR for LVH in patients receiving antihypertensive medications was 0.95 (95% CI, 0.38-2.39, p=0.91).
Table 5.
Multivariable logistic regression analysis of factors associated with LVH
| Estimate | Odds Ratio | Confidence Interval |
P value | |
|---|---|---|---|---|
| Intercept | −2.35 | |||
| Overweight/obese | 1.29 | 3.6 | 1.6-8.0 | 0.002 |
| Anemia | 0.75 | 2.1 | 0.9-4.9 | 0.083 |
| Hypertension | 1.69 | 5.4 | 1.2-23.7 | 0.025 |
| Antihypertensives | 0.98 | 2.7 | 0.8-9.4 | 0.13 |
| Hypertension*Antihypertensives | −1.74 | 0.2 | 0.03-0.99 | 0.05 |
Blood pressure status and allograft function
Allograft function was significantly lower in patients with sustained hypertension than in normotensive patients and those with masked hypertension; no significant difference was found between patients with normal BP and masked hypertension (Table 3). When classified according to antihypertensive medication status (Table 4), there was no significant difference in graft function between normotensive and hypertensive patients (masked and sustained combined) in the group not taking antihypertensive medications. However, patients taking antihypertensive medications had significantly lower eGFR and higher prevalence of eGFR <60 ml/min.1.73m2 than did patients in the group with normal BP or masked hypertension not taking antihypertensive medications. No significant difference in allograft function was found according to the class of antihypertensive medications (ACEI/ARB vs others, data not shown). In a multivariate analysis, hypertension was not associated with allograft function (Table 6). However, use of antihypertensive medications was independently associated with having eGFR <60 ml/min.1.73m2 (OR 3.32, 95% CI: 1.6-6.9, p=0.002). In a sub-analysis, this association remained significant regardless of the class of antihypertensive medications: ACEI/ARB (OR 4.7, 95% CI: 2.0-11.2, p<0.001) or others (OR 2.5, 95% CI: 1.1-5.6, p=0.024).
Table 6.
Multivariable logistic regression analysis of factors associated with eGFR<60 ml/min/1.73m2
| Estimate | Odds Ratio | Confidence Interval |
P value | |
|---|---|---|---|---|
| Intercept | −1.96 | |||
| Rejection | 1.38 | 4.0 | 2.0-7.9 | <0.001 |
| Anemia | 1.03 | 2.8 | 1.4-5.8 | 0.005 |
| Antihypertensives | 1.20 | 3.3 | 1.6-7.0 | 0.002 |
| Hypertension | −0.12 | 0.9 | 0.5-1.6 | 0.699 |
DISCUSSION
This is the largest study in pediatric and young adult kidney transplant recipients to describe BP status based on 24-hour ABPM and to address the potential association of ambulatory hypertension with allograft function and LVH.
This is also one of the first studies to characterize ABP in these patients according to the revised 2014 AHA criteria. Using this classification, 36% of our cohort had masked or sustained hypertension. However, more than a quarter of patients did not fit any BP category and thus were unclassified, representing an important limitation of the AHA guidelines for BP classification in pediatric kidney transplant recipients. Of note, in the only previously published study classifying BP profiles according to the 2014 AHA criteria in this population30, 15% of patients were also found to be unclassified. However, overall BP control in that study, which analyzed patients 5-10 years post their kidney transplant, was worse, with 39% of the patients having sustained hypertension.
Besides high rate of unclassified BP, the current AHA classification is very complicated requiring interpretation of a wake or sleep, systolic or diastolic BP in six different categories. Given these limitations and taking into account the high cardiovascular risk of this population, we simplified the classification into four BP categories by using a lower threshold (24-hour BP load ≥ 25%) as the cutoff to define ambulatory hypertension, a method similarly utilized by the CKiD study in children with CKD10,11. This classification broadens the definition of hypertension on one hand, including both patients with mean ABP > 95th percentile (who have a BP load above 25% anyway) and patients with mean BP lower than the 95th percentile but high BP load. On the other hand, the use of the 24h measurements avoids defining all minor abnormalities (e.g. isolated nocturnal diastolic load >25%) as hypertension. Of note, BP load is not incorporated in the adult AHA guidelines31, but the use of the 25% BP load cutoff was discussed in the recent pediatric AHA guidelines, which concluded that further study is needed to validate this approach.
Regardless of ABP classification, our study confirmed a high prevalence of masked hypertension (25-32%) in children and young adults with a kidney transplant. This is similar to or slightly higher that the prevalence of 19-31%, reported in previous studies14,16,18-20,30 although most of these studies reported a higher prevalence of sustained hypertension. Importantly, even patients taking antihypertensive medications frequently were found to have masked and sustained hypertension, suggesting that hypertension is both underdiagnosed and inadequately treated in this population.
Both masked and sustained hypertension were more frequently seen in African-American or Hispanic patients. While this cross-sectional analysis was not able to evaluate the effect of abnormal ABP on long-term graft function and graft survival, previous studies showing that African-American patients are at a higher risk for poor graft survival32,33 stress the importance of better BP control in these patients.
Previous studies investigating the association of ABP status and cardiac structure in pediatric kidney transplant recipients have reported inconsistent results. Some have found an association of abnormal ABP status with LVMI, 4,13,20,29,34, with others did not confirm this finding17,35,36. Given a large size of study population we were able to stratify patients according to both ABP results and BP treatment and to clarify previous data of a higher prevalence of LVH in patients with controlled hypertension (treated) compared with normotensive patients not receiving BP medications. These results indicate that achieving BP control with antihypertensive medications might not be enough to decrease the risk for LVH. There are a few explanations for these results. First, it is possible that in many subjects antihypertensive agents were initiated relatively recently, thus our data could reflect a delay between the relatively prompt effect on blood pressure normalization and the slower effect on cardiac structure. Alternatively, it may be the case that children who require antihypertensives might need stricter BP control to maintain or achieve normal cardiac geometry than children who do not require antihypertensive need. There also could be additional, unrelated to hypertension mechanisms of cardiac hypertrophy in transplanted patients. Finally, while in children taking antihypertensive medications had similar prevalence of LVH regardless of the level of BP control, it is important to note that the highest frequency of LVH was seen in the small group of patients who had sustained hypertension and were not taking antihypertensive medications.
As in previous studies19,21,22, we found no significant association between ABP status and graft function. However, as in the case of LVH, patients with normal BP who did not require antihypertensive medications had less allograft dysfunction than patients taking antihypertensives, even in those whose BP was within normal levels. This may reflect an effect of previously untreated hypertension on worsening graft function and indicate the need for more aggressive BP control at early stages after transplantation. Interestingly, in a group not treated with antihypertensives, those with masked hypertension had similar graft function to normotensive patients. One possible explanation for this finding is that BP treatment is a surrogate marker for the duration of hypertension. Thus those with masked hypertension and receiving antihypertensive medications may have had a longer duration of uncontrolled BP than those with masked hypertension and not receiving antihypertensive treatment. This is supported by the fact that among those off BP meds with masked hypertension, the median time post-transplant was 2.7 years, while the median time post-transplant in patients on BP meds with masked hypertension was 4.5 years. Unfortunately we did not have information to determine the duration of hypertension prior to ABPM. Another limitation of our study is cross-sectional design so we could not assess a long-term effect of masked hypertension on kidney function and cardiovascular outcomes.
Echocardiographic results were not available for the entire cohort, and some of the available ones were done up to 6 months before/after the ABP measurement; however, there was no substantial difference in demographic and clinical parameters between patients with and without echocardiograms. The variability in the methodology of CBP, echocardiographic measurements, and kidney function determination among participating centers could also affect the results of the study. Despite these limitations, our findings clearly demonstrate that ambulatory hypertension is common and difficult to control in children and young adults after kidney transplantation. The association of ambulatory hypertension with LVH underscores the importance of early recognition of masked hypertension and supports the case for ABPM and cardiac structure evaluation as a part of standard care in these patients.
ACKNOWLEDGMENT
This study was partially presented at 2015 Pediatric Academic Society Meeting, San Diego, CA, USA
FUNDING: This study was funded by the research grant DK 090070 from the National Institutes of Diabetes and Digestive and Kidney Diseases (M.M.M)
ABBREVIATIONS
- ABP
ambulatory blood pressure
- ABPM
ambulatory blood pressure monitoring
- ACEI
angiotensin converting enzyme inhibitors
- AHA
American Heart Association
- ARB
angiotensin receptor blockers
- BP
blood pressure
- CBP
casual blood pressure
- CKD
chronic kidney disease
- CV
cardiovascular
- eGFR
estimated glomerular filtration rate
- LVH
left ventricular hypertrophy
- LVMI
left ventricular mass index
- MWPNC
Midwest Pediatric Nephrology Consortium
- WCH
white coat hypertension
Footnotes
DISCLOSURES: The authors declare no conflicts of interest
AUTHORSHIP
Gilad Hamdani made substantial contributions to study design, analysis and interpretation of data, drafting the manuscript, and approved the final version
Edward J Nehus made substantial contributions to analysis and interpretation of data, drafting the manuscript, and approved the final version
Coral D Hanevold made substantial contributions to collection of data, critical revision of the article for important intellectual content, and approved the final version
Judith Sebestyen made substantial contributions to collection of data, critical revision of the article for important intellectual content, and approved the final version
Robert Woroniecki made substantial contributions to collection of data, critical revision of the article for important intellectual content, and approved the final version
Scott Wenderfer made substantial contributions to collection of data, critical revision of the article for important intellectual content, and approved the final version
David K Hooper made substantial contributions to study design, critical revision of the article for important intellectual content, and approved the final version
Doug Blowey made substantial contributions to collection of data, critical revision of the article for important intellectual content, and approved the final version
Amy Wilson made substantial contributions to collection of data, critical revision of the article for important intellectual content, and approved the final version
Bradley Warady made substantial contributions to collection of data, critical revision of the article for important intellectual content, and approved the final version
Mark M Mitsnefes made substantial contributions to study design, analysis and interpretation of data, drafting the manuscript, and approved the final version
REFERENCES
- 1.Baluarte HJ, Gruskin AB, Ingelfinger JR, Stablein D, Tejani A. Analysis of hypertension in children post renal transplantation--a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Pediatr Nephrol. 1994 Oct;8(5):570–573. doi: 10.1007/BF00858130. [DOI] [PubMed] [Google Scholar]
- 2.The North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Annual Transplant Report. 2010 https://web.emmes.com/study/ped/annlrept/2010_Report.pdf.
- 3.Sinha MD, Kerecuk L, Gilg J, Reid CJ. British Association for Paediatric N. Systemic arterial hypertension in children following renal transplantation: prevalence and risk factors. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012 Aug;27(8):3359–3368. doi: 10.1093/ndt/gfr804. [DOI] [PubMed] [Google Scholar]
- 4.Matteucci MC, Giordano U, Calzolari A, Turchetta A, Santilli A, Rizzoni G. Left ventricular hypertrophy, treadmill tests, and 24-hour blood pressure in pediatric transplant patients. Kidney international. 1999 Oct;56(4):1566–1570. doi: 10.1046/j.1523-1755.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitsnefes MM, Khoury PR, McEnery PT. Early posttransplantation hypertension and poor long-term renal allograft survival in pediatric patients. The Journal of pediatrics. 2003 Jul;143(1):98–103. doi: 10.1016/S0022-3476(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 6.Tutone VK, Mark PB, Stewart GA, et al. Hypertension, antihypertensive agents and outcomes following renal transplantation. Clinical transplantation. 2005 Apr;19(2):181–192. doi: 10.1111/j.1399-0012.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 7.Lezaic V, Naumovic R, Stanic M, et al. Factors affecting graft function in pediatric and adult recipients of adult live donor kidney transplants. Pediatric transplantation. 2007 Dec;11(8):906–913. doi: 10.1111/j.1399-3046.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 8.Group ET, Wuhl E, Trivelli A, et al. Strict blood-pressure control and progression of renal failure in children. The New England journal of medicine. 2009 Oct 22;361(17):1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 9.Minutolo R, Gabbai FB, Agarwal R, et al. Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: a multicenter prospective cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 Nov;64(5):744–752. doi: 10.1053/j.ajkd.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Mitsnefes M, Flynn J, Cohn S, et al. Masked hypertension associates with left ventricular hypertrophy in children with CKD. Journal of the American Society of Nephrology : JASN. 2010 Jan;21(1):137–144. doi: 10.1681/ASN.2009060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuels J, Ng D, Flynn JT, et al. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012 Jul;60(1):43–50. doi: 10.1161/HYPERTENSIONAHA.111.189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma AP, Mohammed J, Thomas B, Lansdell N, Norozi K, Filler G. Nighttime blood pressure, systolic blood pressure variability, and left ventricular mass index in children with hypertension. Pediatric nephrology. 2013 Aug;28(8):1275–1282. doi: 10.1007/s00467-013-2468-x. [DOI] [PubMed] [Google Scholar]
- 13.Calzolari A, Giordano U, Matteucci MC, et al. Hypertension in young patients after renal transplantation: ambulatory blood pressure monitoring versus casual blood pressure. Am J Hypertens. 1998 Apr;11(4):497–501. doi: 10.1016/s0895-7061(97)00484-6. Pt 1. [DOI] [PubMed] [Google Scholar]
- 14.Giordano U, Matteucci MC, Calzolari A, Turchetta A, Rizzoni G, Alpert BS. Ambulatory blood pressure monitoring in children with aortic coarctation and kidney transplantation. The Journal of pediatrics. 2000 Apr;136(4):520–523. doi: 10.1016/s0022-3476(00)90016-7. [DOI] [PubMed] [Google Scholar]
- 15.Seeman T, Dusek J, Vondrak K, et al. Ambulatory blood pressure monitoring in children after renal transplantation. Transplantation proceedings. 2004 Jun;36(5):1355–1356. doi: 10.1016/j.transproceed.2004.04.081. [DOI] [PubMed] [Google Scholar]
- 16.Serdaroglu E, Mir S, Berdeli A. Hypertension and ace gene insertion/deletion polymorphism in pediatric renal transplant patients. Pediatric transplantation. 2005 Oct;9(5):612–617. doi: 10.1111/j.1399-3046.2005.00353.x. [DOI] [PubMed] [Google Scholar]
- 17.McGlothan KR, Wyatt RJ, Ault BH, et al. Predominance of nocturnal hypertension in pediatric renal allograft recipients. Pediatric transplantation. 2006 Aug;10(5):558–564. doi: 10.1111/j.1399-3046.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferraris JR, Ghezzi L, Waisman G, Krmar RT. ABPM vs office blood pressure to define blood pressure control in treated hypertensive paediatric renal transplant recipients. Pediatric transplantation. 2007 Feb;11(1):24–30. doi: 10.1111/j.1399-3046.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 19.Paripovic D, Kostic M, Spasojevic B, Kruscic D, Peco-Antic A. Masked hypertension and hidden uncontrolled hypertension after renal transplantation. Pediatr Nephrol. 2010 Sep;25(9):1719–1724. doi: 10.1007/s00467-010-1552-8. [DOI] [PubMed] [Google Scholar]
- 20.Basiratnia M, Esteghamati M, Ajami GH, et al. Blood pressure profile in renal transplant recipients and its relation to diastolic function: tissue Doppler echocardiographic study. Pediatric nephrology. 2011 Mar;26(3):449–457. doi: 10.1007/s00467-010-1724-6. [DOI] [PubMed] [Google Scholar]
- 21.Gulhan B, Topaloglu R, Karabulut E, et al. Post-transplant hypertension in pediatric kidney transplant recipients. Pediatric nephrology. 2014 Jun;29(6):1075–1080. doi: 10.1007/s00467-013-2721-3. [DOI] [PubMed] [Google Scholar]
- 22.Cameron C, Vavilis G, Kowalski J, Tyden G, Berg UB, Krmar RT. An observational cohort study of the effect of hypertension on the loss of renal function in pediatric kidney recipients. American journal of hypertension. 2014 Apr;27(4):579–585. doi: 10.1093/ajh/hpt140. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology : JASN. 2009 Mar;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2009 Jun;22(6):709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Soergel M, Kirschstein M, Busch C, et al. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. Journal of Pediatrics. 1997 Feb;130(2):178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 27.Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002 Apr;39(4):903–908. doi: 10.1161/01.hyp.0000013266.40320.3b. [DOI] [PubMed] [Google Scholar]
- 28.Flynn JT, Daniels SR, Hayman LL, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014 May;63(5):1116–1135. doi: 10.1161/HYP.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitzmueller E, Vecsei A, Pichler J, et al. Changes of blood pressure and left ventricular mass in pediatric renal transplantation. Pediatric nephrology. 2004 Dec;19(12):1385–1389. doi: 10.1007/s00467-004-1672-0. [DOI] [PubMed] [Google Scholar]
- 30.Tainio J, Qvist E, Miettinen J, et al. Blood pressure profiles 5 to 10 years after transplant in pediatric solid organ recipients. Journal of clinical hypertension. 2015 Feb;17(2):154–161. doi: 10.1111/jch.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005 Feb 8;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 32.Patzer RE, Mohan S, Kutner N, McClellan WM, Amaral S. Racial and ethnic disparities in pediatric renal allograft survival in the United States. Kidney international. 2015 Mar;87(3):584–592. doi: 10.1038/ki.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omoloja A, Mitsnefes M, Talley L, Benfield M, Neu A. Racial differences in graft survival: a report from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Clinical journal of the American Society of Nephrology : CJASN. 2007 May;2(3):524–528. doi: 10.2215/CJN.03100906. [DOI] [PubMed] [Google Scholar]
- 34.Ten Harkel AD, Cransberg K, Van Osch-Gevers M, Nauta J. Diastolic dysfunction in paediatric patients on peritoneal dialysis and after renal transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009 Jun;24(6):1987–1991. doi: 10.1093/ndt/gfp049. [DOI] [PubMed] [Google Scholar]
- 35.Morgan H, Khan I, Hashmi A, Hebert D, McCrindle BW, Balfe JW. Ambulatory blood pressure monitoring after renal transplantation in children. Pediatric nephrology. 2001 Nov;16(11):843–847. doi: 10.1007/s004670100668. [DOI] [PubMed] [Google Scholar]
- 36.Seeman T, Simkova E, Kreisinger J, et al. Control of hypertension in children after renal transplantation. Pediatric transplantation. 2006 May;10(3):316–322. doi: 10.1111/j.1399-3046.2005.00468.x. [DOI] [PubMed] [Google Scholar]