Fig. 3.
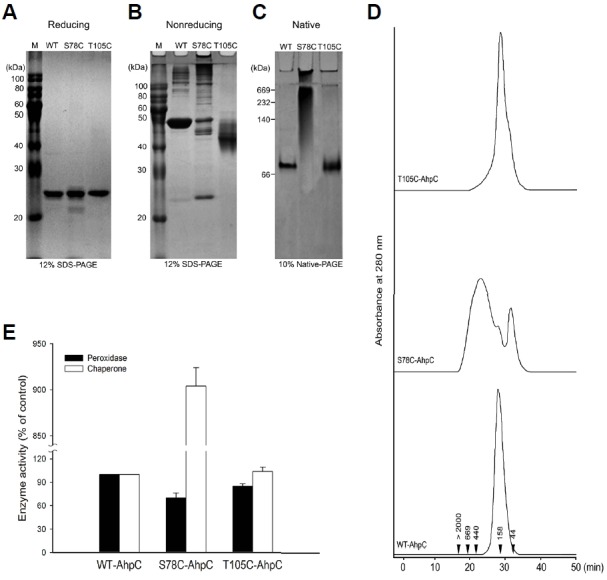
Effect of additional cysteine substitution on the structural and functional change in PaAhpC based on PAGE analysis under reducing (A), non-reducing (B), and native conditions (C) and based on SEC analysis (D). The numbers in the chromatogram represent the molecular weights of the standard proteins: blue dextran (> 2000 kDa), thyroglobulin (669 kDa), ferritin (440 kDa), aldolase (158 kDa), and ovalbumin (44 kDa). (E) Comparison of the enzymatic activities of wild-type and mutant AhpC proteins. The relative activities of the two mutated PaAhpC proteins were compared with the activity of WT-PaAhpC. The peroxidase and chaperone activities of WT-AhpC were set to 100%. Data are the means of at least three independent experiments.
