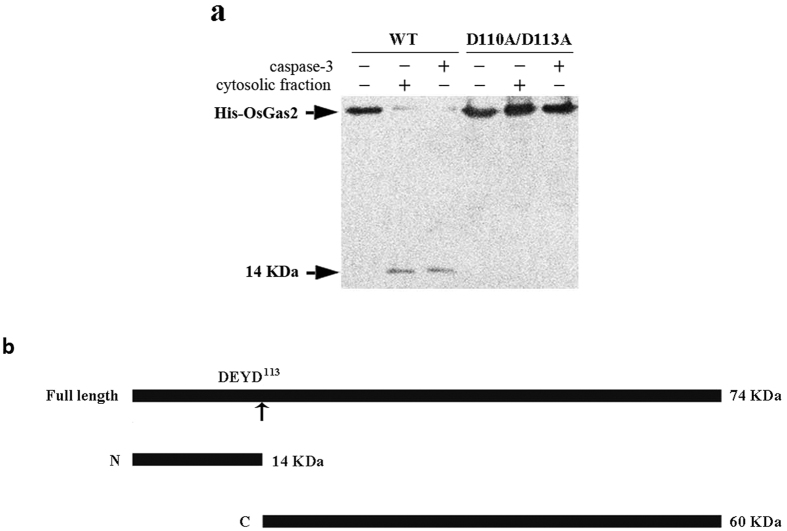
Figure 5. OsGas2 is the substrate of Caspase-3-like activity in rice.
(a) His6-tag was fused at the N-terminus of OsGAS2 and D110A/D113A mutant, and the fusion protein was expressed in E. coli. Purified His6-tagged wild type and mutant protein were incubated respectively with reaction buffer, caspase-3, and cytosolic faction from heat-treated rice suspension cells. After incubation, the reaction products were analyzed by 12.5% SDS-PAGE followed by immuno-blotting with anti-poly histidine antibody that recognized the N-terminal epitope of His6-tagged OsGAS2. Arrows indicated the target protein bands. (b) Schematic for OsGas2 fragmentation by caspase-3. The full length OSGas2 with a MW of 74 KDa is illustrated as a linear molecule with a putative caspase-3 cleavage site DEYD113, which was indicated by an arrow. Digestion with caspase-3 produced N-terminal fragment with MW of 14 KDa (N), and C-terminal fragment with MW of 60 KDa (C).