Abstract
In the eyes of mammals, specialized photoreceptors called intrinsically photosensitive retinal ganglion cells (ipRGC) have been identified that sense photoperiodic or daylight exposure, providing them over time with seasonal information. Detectors of photoperiods are critical in vertebrates, particularly for timing the onset of reproduction each year. In birds, the eyes do not appear to monitor photoperiodic information; rather, neurons within at least 4 different brain structures have been proposed to function in this capacity. Specialized neurons, called deep brain photoreceptors (DBP), have been found in the septum and 3 hypothalamic areas. Within each of the 4 brain loci, one or more of 3 unique photopigments, including melanopsin, neuropsin, and vertebrate ancient opsin, have been identified. An experiment was designed to characterize electrophysiological responses of neurons proposed to be avian DBP following light stimulation. A second study used immature chicks raised under short-day photoperiods and transferred to long day lengths. Gene expression of photopigments was then determined in 3 septal-hypothalamic regions. Preliminary electrophysiological data obtained from patch-clamping neurons in brain slices have shown that bipolar neurons in the lateral septal organ responded to photostimulation comparable with mammalian ipRGC, particularly by showing depolarization and a delayed, slow response to directed light stimulation. Utilizing real-time reverse-transcription PCR, it was found that all 3 photopigments showed significantly increased gene expression in the septal-hypothalamic regions in chicks on the third day after being transferred to long-day photoperiods. Each dissected region contained structures previously proposed to have DBP. The highly significant increased gene expression for all 3 photopigments on the third, long-day photoperiod in brain regions proposed to contain 4 structures with DBP suggests that all 3 types of DBP (melanopsin, neuropsin, and vertebrate ancient opsin) in more than one neural site in the septal-hypothalamic area are involved in reproductive function. The neural response to light of at least 2 of the proposed DBP in the septal/hypothalamic region resembles the primitive, functional, sensory ipRGC well characterized in mammals.
Keywords: photopigment, septum, hypothalamus, thyrotropin-releasing hormone and gonadotropin-releasing hormone-1, thyroid stimulating hormone β and follicle-stimulating hormone β
INTRODUCTION
A large number of mammalian, avian, and other vertebrate species are photoperiodic and thereby display a defined time of year when their reproductive system is activated due to an ability to sense seasonal changes in photoperiod. Several reviews have addressed photoperiodism in birds including its proposed mechanisms, regulation of key annual cycle events, and relevance to productivity and fitness of avian species (Follett and Davies, 1975; Nicholls et al., 1988; Kuenzel, 1993; Foster et al., 1994; Wilson, 1997; Dawson et al., 2001; Sharp, 2005; Ono et al., 2009; Ubuka et al., 2013). The purpose of this paper is to focus on one aspect of the phenomenon, particularly its sensory component. Decades ago it was proposed that vertebrates possess a photo-neuroendocrine system (PNES) composed of 2 components including (1) the photoperiodic axis (receptors and groups of neurons detecting light or changes in photoperiods, and (2) the traditional hypothalamo-pituitary-gonadal (HPG) axis (Scharrer, 1964). Scharrer's first component, the sensory system, will be addressed herein. A neural pathway is required to detect light information from the environment, integrate it and in some manner connect with the classical HPG axis to regulate reproductive function at the appropriate time each year. Significant progress in birds and mammals has been made in identifying receptors, neurons, and hormones activated early by photoperiodic induction. In mammals, a neural pathway involving specialized receptors containing a primitive-type photopigment has been determined that is designed for detecting irradiance or nonimage light stimulation. The general, mammalian neural model that couples light-dark daily cycles to development of the reproductive system will first be reviewed. Thereafter, a remarkable, conservative system discovered in both mammals and birds will be highlighted. It involves thyroid hormones and an endocrine organ, the pars tuberalis, located adjacent to the anterior pituitary gland. How the sensory photopigment/receptor system and thyroid hormone converting pars tuberalis/mediobasal hypothalamic complex are coupled for the regulation of the HPG axis in birds remains controversial. Finally, an avian neural sensory pathway regulating reproduction will be proposed based on recent data from the literature and our laboratory as well as areas where gaps in our knowledge remain regarding the regulation of this critical neuroendocrine system.
MAMMALIAN NEURAL PATHWAY REGULATING SEASONAL REPRODUCTION
(1). Sensory Photopigment System Involving the Eyes, Suprachiasmatic Nucleus, and Pineal Gland
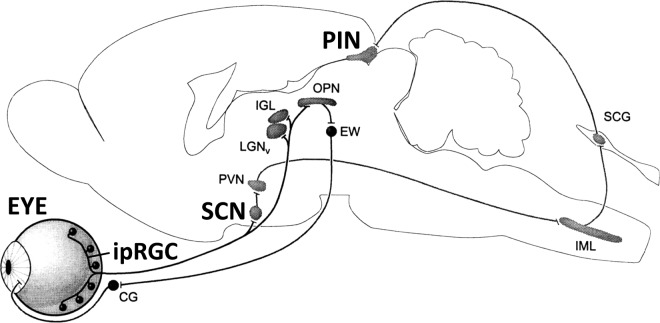
In mammals, a seminal discovery showed that specialized neurons exist in the retina of mammals that function to detect nonimage ambient light intensity. Specifically, intrinsically photosensitive retinal ganglion cells (ipRGC) in the eyes were found responsible for detecting photoperiodic information and synchronizing circadian rhythms (Berson et al., 2002; Berson, 2003). Rods and cones are predominant receptors in the eyes, whereas retinal ganglion cells (RGC) are usually regarded as output neurons of the retina and function commonly to transmit visual, image-type information to the brain. A specialized, unique class of RGC called ipRGC, however, has been shown to be a small population of RGC containing a novel opsin, melanopsin (Opn4; Provencio et al., 1998, 2000; Berson et al., 2002; Hannibal et al., 2002), and therefore they function as receptors. Electrophysiological evidence showed that in contrast to cones and rods that rapidly hyperpolarize following light stimulation, ipRGC were shown to depolarize and responded with a delayed and very slow, gradual response following photostimulation (Berson et al., 2002). The neural pathway from ipRGC was documented to be a slow but sustained electrophysiological response (Berson et al., 2002) that was carried to its first target, the suprachiasmatic nucleus, regarded as the master clock responsible for regulating circadian rhythms. The pathway was previously shown to be a specialized retinohypothalamic tract (Moore et al., 1995), and neural output from the suprachiasmatic nucleus was thereafter mapped to the paraventricular nucleus (PVN). The PVN in turn was shown to project monosynaptically to the origin of the sympathetic nervous system, the intermediolateral nucleus located in the spinal cord. In the cervical region of spinal cord, a small group of neurons project from the intermediolateral to the superior cervical ganglion and from that ganglion to the pineal gland (Figure 1; Berson, 2003). A nocturnal melatonin signal produced by the pineal has been documented to provide the photoperiodic message and the melatonin signal is phased to the light-dark cycle by retinal input from the ipRGC. Ultimately, photoperiodic changes in melatonin secretion control the mammalian gonadal neuroendocrine pathway (Hazlerigg and Loudon, 2008).
Figure 1.
Sagittal view of the mammalian brain. Intrinsically photosensitive retinal ganglion cells (ipRGC) project to the suprachiasmatic nucleus (SCN), paraventricular nucleus (PVN), and origin of the sympathetic arm of the autonomic nervous system called the intermediolateral nucleus (IML). The IML projects to the superior cervical ganglion (SCG) followed by the pineal gland (PIN). This pathway regulates melatonin release by the pineal and can provide seasonal information to animals (see text). CG, ciliary ganglion; EW, Edinger-Westphal nucleus; IGL, intergeniculate leaflet; LGNv, ventral division of lateral geniculate nucleus; OPN, olivary pretectal nucleus, structures involved in the pupillary light reflex (modified from Berson, 2003). Reprinted from Trends in Neurosciences, volume 26, D. M. Berson, Strange vision: Ganglion cells as circadian photoreceptors, pages 314–320, copyright 2003, with permission from Elsevier..
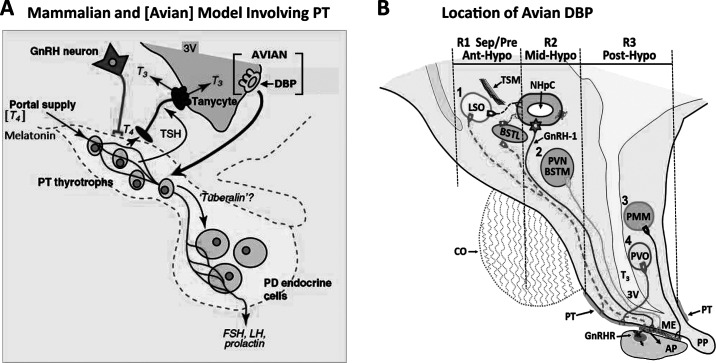
Species differences for the target site(s) of melatonin have been shown among mammalian species. Of relevance, melatonin does not appear to act directly on gonadotropin-releasing hormone-1 (GnRH-1) neurons (Lehman et al., 1997; Malpaux et al., 1998). A major target appears to be the pars tuberalis (PT) based on binding sites present with greatest density in that organ; however, hypothalamic and other sites were also identified (Bittman and Weaver, 1990). Evidence suggests the PT is a major target of melatonin in mammalian species, particularly Soay sheep due to the presence of type 1 melatonin (MT1) receptors (Hazlerigg and Loudon, 2008). Nonetheless, studies in some sheep and hamsters suggest that the PT does not mediate the action of melatonin on the neuroendocrine reproductive axis because melatonin microimplants placed in the mediobasal hypothalamus or third ventricle stimulated luteinizing hormone (LH) release (Malpaux et al., 2001). Hence, it has been hypothesized that a complex network of interneurons containing melatonin receptors exists within the mammalian mediobasal hypothalamus and projects to GnRH neurons in the preoptic area (Malpaux et al., 2001). Noteworthy is that the PT, in addition to having MT1 receptors, also has thyroid-stimulating hormone (TSH) producing cells that, when TSH gene expression is induced, result in activation of an enzyme type 2 deiodinase (DIO2). The enzyme DIO2 is responsible for converting thyroxine (T4) to its active form 3,5,3′ triiodothyronine (T3) and T3 accumulates not only in the third ventricle but also in the brain parenchyma as shown in Figure 2A (Leonard, 1988; Hanon et al., 2008). This highly conserved PT and mediobasal hypothalamic complex involving thyroid hormones is discussed in the next section.
Figure 2.
A. Unifying model for pars tuberalis (PT)-dependent photoperiodic regulation of seasonal endocrine function in mammals and birds. Within brackets are deep brain photoreceptors (DBP) found in avian species not mammals. Additionally, T4 shown in brackets is available to the PT in both mammals and birds via the portal blood supply, cerebrospinal fluid, or both, whereas melatonin influences PT function in mammals (see text). FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; PD, pars distalis; PT, pars tuberalis; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone; 3V, third ventricle (modified from Hazlerigg and Loudon, 2008). Reprinted from Current Biology, volume 18, D. Hazlerigg and A. Loudon, New insights into ancient seasonal life timers, pages R795–R804, copyright 2008, with permission from Elsevier. B. Septal-hypothalamic area of birds. Four loci have been proposed to contain DBP: (1) lateral septal organ (LSO) including the lateral bed nucleus of the stria terminalis (BSTL), (2) paraventricular nucleus and medial bed nucleus of the stria terminalis complex (PVN/BSTM); (3) premammillary nucleus (PMM), and (4) paraventricular organ (PVO). The 3 nearly rectangular regions show areas dissected for real-time reverse-transcription PCR: region 1 (R1) Sep/Pre/Ant-Hypo, septal preoptic anterior hypothalamic area; region 2 (R2) Mid-Hypo, middle hypothalamic area; region 3 (R3) Post-Hypo, posterior hypothalamic area. AP, anterior pituitary; CO, optic chiasma; GnRH-1, type 1 gonadotropin-releasing hormone; GnRHR, GnRH receptors; ME, median eminence; NHpC, nucleus of the hippocampal commissure; PP, posterior pituitary; TSM, septopallial mesencephalic tract. Color version available in the online PDF.
(2). Highly Conserved Thyroid Hormone Converting System Consisting of the PT and Mediobasal Hypothalamus in Mammals and Birds
Thyroid hormones, T4, and its active form, T3, have been shown to be critical for affecting seasonal physiological events in the annual cycles of vertebrates, particularly reproductive function. For example, thyroxine has been shown to be required for termination of breeding behavior and reproductive function in birds (Nicholls et al., 1988; Wilson and Reinert, 1995, 2000; Wieselthier and Van tienhoven, 1972) as it functions to make birds photorefractory to long-day photostimulation and initiates molt (reviewed in Kuenzel, 2003; Kuenzel et al., 2005). Thyroxine similarly functions in terminating the breeding season of mammals, most of which respond reproductively to short-day photoperiods (Billings et al., 2002). A supply of T4 from the thyroid and melatonin from the pineal enters the cardiovascular system of vertebrates, particularly mammals, and is transported to the anterior pituitary and the PT via the portal capillary system (Figure 2A, modified from Hazlerigg and Loudon, 2008).
A fundamental system has recently been discovered showing a highly conservative system in both mammals and birds (Figure 2A). A central component is the PT, which contains specialized cells that synthesize TSH. In addition, cells of the PT or the mediobasal hypothalamus contain 2 enzymes, DIO2 and deiodinase type 3 (DIO3); the former converts T4 to its more bioactive form T3, whereas DIO3 inactivates T3 to 3,3′-diiodothyronine (T2) as well as converts T4 to yet another more inactive form, reverse T3, in mammals (Lechan and Fekete, 2005) as well as birds (Van der Geyten et al., 2005; Yasuo et al., 2005). In addition, specialized glial cells, tanycytes, exist at the base of the third ventricle proposed to facilitate communication between the cerebrospinal fluid within the third ventricle of the brain and the pars distalis (anterior pituitary) and portal vasculature (Rodriguez et al., 2005). In Japanese quail, a T4-specific transporter gene Oatp1c1 as well as Dio2 and Dio3 genes have been expressed in the mediobasal hypothalamus and PT (Nakao et al., 2006; Yasuo and Yoshimura, 2009), suggesting strongly that because both birds (Yasuo and Yoshimura, 2009) and mammals (Yasuo and Korf, 2011) have the genes as well as anatomical structures, tanycytes, in and adjacent to the median eminence, the 2 classes of vertebrates have the components that could enable a retrograde pathway to exist whereby the endfeet of tanycytes that contact the PT or portal blood vessels, and their cell bodies that lie immediately adjacent to the third ventricle could provide that communication between the peripheral blood/PT and the cerebrospinal fluid of the central nervous system. Specifically, T4 can be taken up from the blood into the PT and perhaps by transcytosis and a retrograde pathway via tanycytes, the essential molecules (T4 and TSH can be transported from the PT to the basal region of the third ventricle where cell bodies of tanycytes occur that have been shown to contain TSH receptors (Nakao et al., 2008; Yasuo and Korf, 2011). The binding of TSH to the TSHR results in stimulation of DIO2 and inhibition of DIO3 resulting in increased T3 production (Yasuo et al., 2005; Ono et al., 2009; Yasuo and Korf, 2011). Of relevance is that T3 injected into the third ventricle of the brain resulted in rapid development of the testes in American tree sparrows (Wilson and Reinert, 2000) and Japanese quail (Yoshimura et al., 2003). Similarly, TSH injected intracerebroventricularly likewise increased size of testes and was shown to induce expression of the Dio2 gene (Nakao et al., 2008).
In mammals and birds, it is unclear how integration of responses of the PT and mediobasal hypothalamic areas by changes in thyroid hormone and melatonin levels activate/deactivate GnRH-1 neurons of the HPG axis. Evidence presented to date for the regulation of GnRH-1 hormonal secretions involves an intriguing mechanism based on confocal and electron microscopic data showing that the terminals of GnRH neurons become exposed in the median eminence by retraction of glia surrounding their axon endings, allowing GnRH-1 secretions to flow into the median eminence and carried by the portal capillaries to the anterior pituitary. There is no question that elegant findings in the rat (King and Rubin, 1994; Prevot et al., 2010) and Japanese quail (Yamamura et al., 2004, 2006) have shown an important functional relationship of the terminal field of GnRH neurons and surrounding glial processes in the median eminence. Prior to the preovulatory surge of LH in the rat and after transfer of Japanese quail to long-day photostimulation, retraction of specialized glial processes around GnRH terminals has been demonstrated, suggesting strongly that glia participate in the modulation of GnRH secretion into the portal vasculature within the median eminence. An alternative hypothesis in mammals is that melatonin receptors exist not only in the PT but also in the basal hypothalamus and this hypothalamic neuronal plexus may be a critical site that interacts with GnRH neurons (Malpaux et al., 2001). The issue of whether there are other neural sites in the brain that project directly to the cell bodies or perikarya of GnRH-1 neurons to regulate their function rather than solely controlling GnRH-1 secretions at the terminal sites of these neurons in the median eminence will be addressed in the next section.
AVIAN NEURAL SENSORY PATHWAY REGULATING SEASONAL REPRODUCTION
The avian neural sensory pathway regulating gonadal development, similar to mammals, has 2 major parts; however, the initial (1) sensory, photopigment system, is very different. In contrast the second part, (2) highly conserved thyroid hormone converting system comprising the PT and mediobasal hypothalamus, is quite similar to that of mammals. The avian neural system and its 2 components will be briefly described in the next 2 sections and end with an issue of how the 2 parts of the avian sensory system communicate.
(1). Sensory Photopigment System Involves Deep-Brain Photoreceptors
In contrast to mammals, past data suggest that the neural pathway regulating the reproductive system in birds is different, particularly with the initial component of the pathway involving photoreceptors. Specifically, the eyes (Menaker et al., 1970; Harrison, 1972; Oishi and Lauber, 1973; Follett and Davies, 1975; McMillan et al., 1975; Wilson, 1989, 1991), suprachiasmatic nucleus (Davies and Follett, 1975), and pineal gland (Harrison and Becker, 1969; Homma and Sakakibara, 1971; Siopes and Wilson, 1974; Gwinner, 1981; Wilson, 1991) have not been found to be critical components for transducing photoperiodic information into an appropriate behavioral and physiological response for successful reproduction at the proper season each year as has been found in mammals (Figure 1). The primary sensory system shown to detect photoperiodic cues is thought to be nonretinal, nonpineal deep brain photoreceptors (DBP; Benoit, 1964; Wilson, 1991; Foster et al., 1994; Vigh et al., 2002).
Currently 4 sites in the avian brain have been proposed to house DBP (Figure 2B): 1) the lateral septal region including the lateral septal organ (LSO; Silver et al., 1988; Kuenzel, 1993; Kuenzel and Blähser, 1994; Saldanha et al., 1994; Kuenzel et al., 1997; Wada et al., 2000; Saldanha et al., 2001; Li et al., 2004; Chaurasia et al., 2005; Rathinam and Kuenzel, 2005; Li and Kuenzel, 2008), 2) the PVN (Soni and Foster, 1997; Tomonari et al., 2007; Halford et al., 2009; Davies et al., 2012), 3) premammillary nucleus (PMM; Kang et al., 2007, 2009, 2010; Thayananuphat et al., 2007; El Halawani et al., 2009; Kosonsiriluk et al., 2013), and 4) the paraventricular organ (PVO; Vigh-Teichmann et al., 1980; Vigh and Vigh-Teichmann, 1998; Nakane et al., 2010; Nakane and Yoshimura, 2010). It could be that all 4 brain areas contain DBP and are functionally coordinated to ensure continual function of the essential detection of photoperiodic information. To date, however, which sites are critical and how they communicate among themselves remain unknown. Each of the 4 neural loci proposed to house DBP is in different brain structures, and 3 distinct photopigments have been discovered and thought involved in the reception of photoperiodic information: melanopsin/Opn4 (Chaurasia et al., 2005; Kang et al., 2010), neuropsin/Opn5 (Nakane et al., 2010; Ohuchi et al., 2012), and vertebrate ancient opsin (VAOpn; Foster et al., 1985, 1994; Halford et al., 2009; Davies et al., 2012).
(2). Highly Conserved Thyroid Hormone Converting System Consisting of the PT and Mediobasal Hypothalamus in Mammals and Birds
As discussed previously in the Mammalian Neural Pathway Regulating Seasonal Reproduction section, specifically its second section, the Highly Conserved Thyroid Hormone Converting System, is remarkably similar to that in birds. The major difference in the mammalian system is that the 2 part sensory-converting system is linked by the production of the hormone melatonin by the pineal gland. An issue that remains unanswered is how the 2 parts are linked in birds to drive the reproductive system.
A MISSING LINK BETWEEN (1) THE AVIAN SENSORY PHOTOPIGMENT SYSTEM (DBP) AND (2) THE HIGHLY CONSERVED THYROID HORMONE CONVERTING SYSTEM INVOLVING THE PT AND MEDIOBASAL HYPOTHALAMUS
In the mammalian system, the final structure in the sensory photopigment system is the pineal gland that produces the hormone melatonin during the dark period. The primary structure of the second part, the conserved thyroid hormone converting system is the PT, and in mammals, it is known to have MT1 receptors (Dardente et al., 2003). Melatonin is therefore the diurnal indicator that can signal day lengths throughout the year and link that information to the thyroid hormone converting system located in the PT to influence the production and transport of T3 into the brain.
In birds, the pineal is not essential for development of the gonads nor have melatonin receptors been reported in the avian PT. A major question to address is what links the avian DBP to the conserved thyroid hormone converting system in the PT. The current model of the avian PNES (Figure 2A) shows DBP located at the base of the third ventricle in the paraventricular organ, one of the proposed sites containing DBP and the photopigment Opn 5 (see brackets marked avian in Figure 2A) projecting to cells in the PT. As the diagram shows, the PT structure is a distinct region of the anterior pituitary (Morgan and Williams, 1996) and therefore has secretory cells such as TSHβ (Yasuo et al., 2005). Just as some mammals have MT1 receptors in the PT to synchronize the day-night cycle from ipRGC to PT function, one would expect that receptors for the specific neuromodulators released by special avian DBP neurons should be located on PT cells to activate their production of TSHβ (Figure 2A). To the best of our knowledge, receptors on cells within the avian PT associated with seasonal function, particularly gonadal development, have not been reported.
The second concern involves the major manner in which GnRH-1 neurons are regulated (Figure 2A). Evidence presented to date for the regulation of GnRH-1 hormonal secretions involves a mechanism discussed previously [under subheading (2) Highly Conserved Thyroid Hormone Converting System…] showing that the terminals of GnRH neurons become exposed in the median eminence by retraction of glia surrounding their axon endings, allowing GnRH-1 secretions to flow into the median eminence upon either a transfer of birds to long-day photostimulation or central administration of T3. Indeed that evidence supports a role for glia in modulating GnRH secretion into the portal vasculature. Nonetheless, a neural circuit involving the dendrites and cell bodies of GnRH-1 neurons is critical for the required several fold changes in GnRH-1 gene expression during the reproductive season. Therefore, the specialized avian DBP neurons that detect photoperiodic information should be part of a key neural circuit with connectivity to GnRH-1 neurons, thereby having enhanced ability to regulate output from the HPG axis.
DATA SUPPORTING INTRINSICALLY PHOTOSENSITIVE DBPS IN THE LSO AND MULTIPLE BRAIN LOCI CONTAINING FUNCTIONAL DBP
Functional Electrophysiological Features of Avian DBP
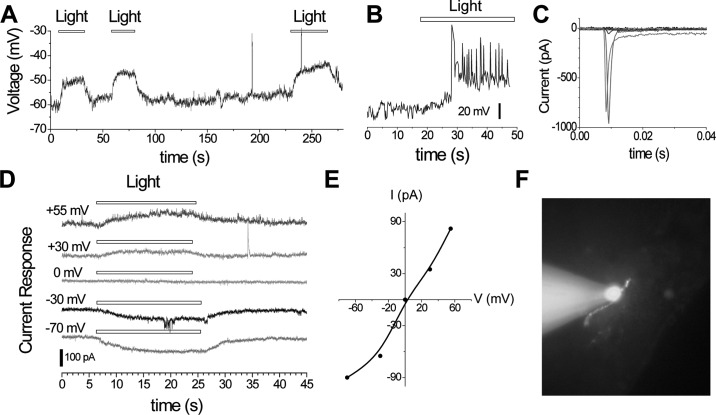
We chose to examine the LSO with the intention of patch-clamping neurons in that structure as we were familiar with its location and could identify its unusual cerebrospinal fluid contacting neurons (CSFcn) in unstained slices of chick brain (Kuenzel and Blähser, 1994; Kuenzel et al., 1997). Previous data showed that when the LSO region was bilaterally lesioned in chicks followed by transferring birds from a short to a long photoperiod, the experimental group showed a significant attenuation of gonadal development compared with sham-operated controls (Rathinam and Kuenzel, 2005). Additionally, neurons in the septum have been shown to contain Opn4 (Chaurasia et al., 2005) and vasoactive intestinal polypeptide (VIP), the latter showing gene activation following photostimulation (Li and Kuenzel, 2008). Evidence suggested the presence of DBP in this brain region. Brains were rapidly removed from chick embryos on d 18 to 20 (ED18–20) and sectioned at 200 to 300 μm using a vibratome. The brain and slices were kept viable in artificial cerebrospinal fluid, which was constantly oxygenated with 95% O2 and 5% CO2. Slices were placed in a recording chamber and viewed under an upright fluorescence microscope with a 40× water immersion objective lens. Infrared differential interference contrast optics were used to visualize neurons through an infrared-sensitive charge-coupled devicecamera. To target neurons of interest for recording in the LSO, landmarks characteristic of the lateral edge of the septal region at the base of the lateral ventricle were identified under infrared illumination. Cells having a perikaryon greater than12 μm in diameter were targeted for whole-cell patch-clamp recording to increase the chance of recording from neurons rather than glia. The recording pipette contained a physiological intracellular solution supplemented with a fluorescent dye (Alexa Fluor 594, 0.25 mM). During whole-cell patch clamp recording, the fluorescent dye was diffused into each cell, thus allowing morphological identification of cell type and axon projection sites at the end of recording (under epi-fluorescence illumination) as described in detail (Lee and Zhou, 2006; Zhou and Lee, 2008). The first objective was to determine the resting membrane potential and voltage response of neurons in the LSO to photostimulation. The resting membrane potential was measured in darkness in the current-clamp mode with zero holding current and found to be around −60 mV (Figure 3A). When photostimulated, cells were depolarized by about 8 to 12 mV (Figure 3A). In most cells, the depolarization had a slow rising phase and remained subthreshold (Figure 3A). Occasionally, however, the light-evoked slow depolarization was strong enough to generate supra-threshold action potentials, resulting in delayed spikes (Figure 3B). Voltage-gated Na currents were also detected from these cells (Figure 3C). When recorded under voltage clamp at various holding potentials, these cells responded to light with slowly developing current responses (Figure 3D) that reversed direction at approximately 0 mV, suggesting that they were mediated by nonselective cation channels. The current-voltage (I-V) relationship plotted in Figure 3E shows a near linear relationship. When examined under fluorescence microscopy, all 6 light-responsive cells recorded to date showed a characteristic bipolar morphology as presented in Figure 3F (unpublished data, Z. J. Zhou and W. J. Kuenzel).
Figure 3.
A. Light-evoked responses from a neuron in the lateral septal organ, showing slowly rising subthreshold depolarization under current clamp. B. Light-evoked response from another cell under current clamp, showing a light-evoked slow depolarization, which led to delayed suprathreshold spikes. C. Voltage-gated Na currents (shown after leak subtraction) from the same cell as in B, in response to a series of depolarizing voltage steps of increasing amplitude from a holding potential of −70 mV. D. Light-evoked current responses recorded at various holding potentials under voltage clamp, showing a reversal potential near 0 mV. E. Current: voltage (I-V) relation of light evoked currents in D. F. Digital image of an Alexa Fluor 594-filled neuron near the ventricular surface of a septal slice under whole-cell patch showing a bipolar morphology. The intensity of full-field light stimulation was 0.04 nW µm−2. Color version available in the online PDF.
Electrophysiological data (Figure 3) suggest that either the recorded bipolar cells or neurons presynaptic to those cells are DBP. Notably, the slowness (approximately 5 s in rise time) of the response, and most interestingly, its polarity (depolarization, rather than the characteristic hyperpolarization found in rods and cones) following light onset, amplitude of the subthreshold depolarization in response to light (5–20 mV) and the cation-selective nature of the underlying ion channels all share a remarkable similarity with the ipRGC in the mammalian retina (Berson et al., 2002; Berson, 2003). The seminal data of Berson et al., (2002) and others (Moore et al., 1995; Provencio et al., 2000; Hannibal et al., 2002; Hattar et al., 2002) have shown that the mammalian ipRGC is a primitive form of receptor and has been proposed to use an invertebrate-like phototransduction cascade (Qiu et al., 2005). Similarly, the data suggest that neurons in the LSO of chicks contain photoreceptors with similar electrophysiological responses to light as found in the mammalian ipRGC.
Unknown at this time is what photopigment is contained in the light responsive bipolar cells within the LSO of chicks. Importantly, synaptic blockers to the bipolar neurons are needed to ascertain whether the cells recorded are truly intrinsically photosensitive or whether cells upstream from these neurons are the DBP in this brain region. Additionally, because there are 3 other brain regions proposed to have DBP, each of the sites will require a detailed examination of their neurons to determine if their electrophysiological characteristics resemble those shown in Figure 3.
A paper has just been published showing that cerebrospinal fluid contacting neurons (CSFcn) in the PVO (locus #4, Figure 2B) have been patch-clamped, shown to contain a mammalian form of Opn5, and likewise showed depolarization following light stimulation. Importantly, after major synaptic inputs were blocked with application of a cocktail with antagonists of major neurotransmitters, the depolarized response persisted after photostimulation. Results are strong evidence that the CSFcn in the PVO are intrinsically photosensitive (Nakane et al., 2014).
Because there are currently 2 distinct neuroanatomical loci, the LSO and PVO, with electrophysiological evidence for the existence of DBP, an experiment was therefore designed to determine whether additional functional data could be obtained regarding whether more than one site in the septal and diencephalic regions of the bird contain DBP. Importantly, an attempt was made to determine if genes associated with the HPG axis were activated along with photopigment genes associated with proposed DBP in the avian brain. A set of genes was selected to determine their change in expression over 3 time periods to obtain evidence when photopigments and the HPG axis showed a significant response following the transfer of birds from a short photoperiod to long-day photostimulation.
Multiple Brain Sites Containing Functional DBP
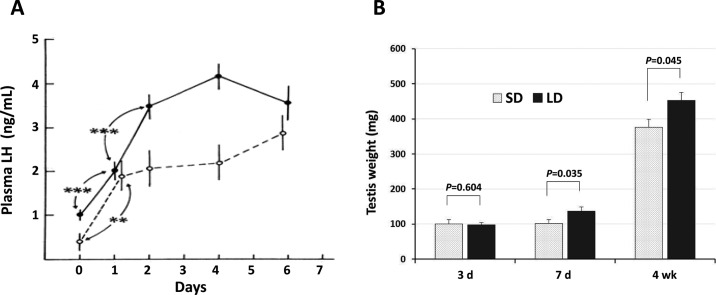
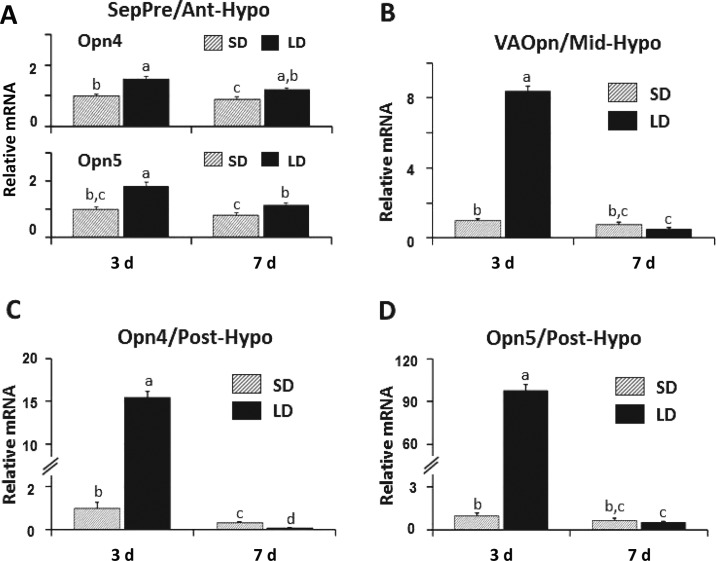
Chickens are long-day breeders, meaning that their reproductive organs develop rapidly when birds are placed in an environment with long photoperiods, usually ≥14 h of light each day. To determine if any of the 4 distinct neural structures proposed to contain DBP showed changes in gene expression following long-day photostimulation, the following experiment was designed. Male chicks, Cobb 500 broilers, were raised in an environment with a short daily period of 8 h of light and 16 h of dark (8L:16D), beginning on d 3 after hatch until 2 wk of age. Birds were then transferred to a stimulatory long day (16L:8D) photoperiod and sampled 3, 7, and 28 d later while controls were maintained on 8L:16D for the total 6-wk period. The average light intensity in each room was set at 55 lx. Brains were removed rapidly, frozen, and the septal-hypothalamic regions were dissected into 3 regions: (R1) the septal, preoptic, anterior hypothalamic (SepPre/Ant-Hypo) region including the LSO and the lateral bed nucleus of the stria terminalis (BSTL; Figure 2B); (R2) the mid-hypothalamic (Mid-Hypo) region containing the PVN and a major group of GnRH-1 neurons surrounding the nucleus of the hippocampal commissure (Figure 2B); (R3) the posterior hypothalamic (Post-Hypo) region containing the PMM and PVO, PT, and median eminence, Figure 2B). The reason for making the 3 dissections is that the first region includes the LSO and lateral septum with scattered neurons containing Opn4 (Chaurasia et al., 2005) and the BSTL with Opn5 neurons (Ohuchi et al., 2012). The Mid-Hypo contains VAOpn (Halford et al., 2009) neurons in the PVN and medial bed nucleus of the stria terminalis. The Post-Hypo region (Figure 2B) contains Opn4 neurons in the PMM (Kang et al., 2010) and Opn5 neurons in the PVO (Nakane et al., 2010). In addition, the anterior pituitary was dissected and frozen while the testes were sampled and weighed. Frozen brain regions and pituitary were extracted for RNA and prepared for real-time reverse-transcription PCR. Sampling d 3 was selected as previous data with adult Japanese quail showed that transferring birds from a short to a long photoperiod resulted in significant elevation of plasma LH produced by the anterior pituitary in 24 h with peak release at 4 d (Follett and Davies, 1975; Figure 4A). Therefore sampling before d 4 should provide the opportunity to obtain a peak response for gene expression of brain photopigments. Days 7 and 28 were selected to determine if significant changes in gene expression levels persist. Results showed that Opn4 and Opn5 in the SepPre/Ant-Hypo of long-day birds showed significant increases in gene expression compared with controls on d 3 and they persisted when sampled on d 7 (Figure 5A). In the Mid-Hypo region, VAOpn showed a highly significant increase in the long-day group on d 3. Of interest, no significant changes were detected at 7 (Figure 5B) and 28 d (data not shown). Unexpectedly, extremely high gene expression for Opn4 and Opn5 were obtained for the long-day birds in the Post-Hypo brain region (Figure 5C,D). In contrast, the mRNA for each of the photopigments in the Post-Hypo region was nearly undetectable for the long-day groups at 7 and 28 d (data not shown). In summary, the SepPre/Ant-Hypo region that contains 1 of the 4 proposed neuroanatomical candidate sites with DBP, indicated as locus (#1) LSO/BSTL in Figure 2B was the only region that showed a significant elevation for Opn4 and Opn5 that persisted for sampling d 3 and 7 in the long-day treatment birds, albeit the responses obtained were less than double that of controls (Figure 5A). The Mid-Hypo region that contains locus #2 (PVN/medial bed nucleus of the stria terminalis complex), displayed more than an 8-fold increase in VAOpn mRNA transcripts by the long-day group (Figure 2B, 5B) for the d 3 sampling period. No increase in gene expression was observed by the long-day group for d 7 or 28. Of interest, the PVN has also been shown to be the major source of gonadotropin inhibitory hormone containing neurons (Ukena et al., 2003). The Post-Hypo dissected region, containing locus #3 (PMM) and locus #4 (PVO, Figure 2B) showed dramatic increases in gene expression for Opn4 and Opn5 on d 3. The Opn4 increased over 15-fold, whereas Opn5 increased 95-fold (Figure 5C, D). Curiously in sampling d 7 and 28, mRNA for the Opn4 and Opn5 in the long-day treatment group was barely detectable (Figure 5C, D; d 28 data not shown; unpublished data, S. W. Kang and W. J. Kuenzel).
Figure 4.
A. Adult Japanese quail (solid lines and circles) and adult white-crowned sparrows (dashed lines) were transferred from short to long day lengths starting on d 0. Plasma luteinizing hormone (LH) of Japanese quail significantly increased on d 1 and peaked on d 4 (Follett and Davies, 1975). **P < 0.05; ***P < 0.01. B. Weight of testes of broiler chicks transferred from short day (SD) to long day (LD) and measured on d 3, 7, and 28. Weight of testes was significantly increased on d 7 and 28 in LD birds compared with SD controls (P ≤ 0.05).
Figure 5.
Gene expression of photopigments in the 3 septal/diencephalic regions. A. Opsin 4 (Opn4) and Opsin 5 (Opn5) showed sustained increases in mRNA levels in the septal, preoptic, anterior hypothalamic region (SepPre/Ant-Hypo) following long-day (LD) photoperiods on d 3 and 7. B. Vertebrate ancient opsin (VAOpn) displayed an 8-fold increase in gene expression in mid-hypothalamus (Mid-Hypo) on d 3 of LD photostimulation with low levels of gene expression on d 7 and 28 for both experimental groups. C and D. The Opn4 and Opn5 showed extremely high levels of gene expression, respectively, in the posterior hypothalamus (Post-Hypo) on d 3 of LD photostimulation. Low levels of gene expression were displayed for both Opn4 and Opn5 on d 7 and 28 for both the short-day (SD) and LD groups (unpublished data, S. W. Kang and W. J. Kuenzel). Different letters (a–d) above columns in each figure are significantly different (P < 0.05).
What is the significance of the extremely high levels of expression for Opn4, Opn5, and VAOpn for only the long-day treatment group during sampling d 3? Does this mean that all 3 photopigments are important and yet they only have a single time period of expression for acknowledging that a long-day photoperiod has occurred? A relevant paper appeared showing that in Soay sheep that were transferred from a short day of 8L:16D to a long day of 16L:8D resulted in a significant increase in TSHβ on the third day of long-day photostimulation (Dardente et al., 2010). They have uncovered what they believe is a group of transcription coactivators including circadian transcription factor thyrotrope embryonic factor (Tef), eyes absent 3 (Eya3) and sine oculis (Six) gene family. It has been proposed that the proteins produced by the 3 genes form a transcriptional coactivator complex that binds to the promoter region of the TSHβ gene and functions as a conserved driver activating a molecular switch composed of TSHβ and DIO2 found in the pars tuberalis (Dardente et al., 2010). In birds, it could be that a dramatic increase in gene expression for all 3 photopigments is sufficient to activate a similar cascade for developing the reproductive system. Indeed it has been shown that a single, pharmacological dose of thyroid hormone has a marked effect on avian seasonal, physiological events. Specifically a peripheral injection of T4 not only stimulates reproductive development but also initiates the development of photorefractoriness (subsequent loss of response to long-day photostimulation) when injected during the time birds were exposed to long photoperiods (Goldsmith and Nicholls, 1992; Wilson and Reinert, 1995). Wilson and Reinert (1995) also proposed that a single injection of thyroxine exerted organizational-like effects that appeared to program seasonal reproduction and photorefractoriness. It is not unreasonable to suggest that in birds a significant increase in gene expression for photopigments/DBP coupled closely with the previously suggested transcription coactivators serving as a driver could subsequently stimulate a molecular switch located in the PT complex (Dardente et al., 2010) to program seasonal reproduction.
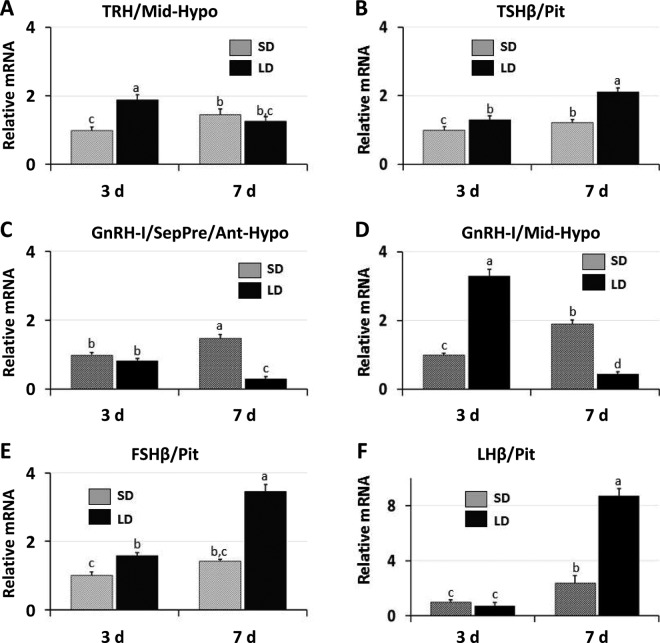
To determine whether a sequence of molecular events can be ascertained between photopigment/DBP activation and pituitary increase in gonadotropins, the following neuroendocrine data were monitored, including the gonadotropic and thyrotropic axes. Specifically gene expression for GnRH-1 was examined in the SepPre/Ant-Hypo and Mid-Hypo regions and thyrotropin-releasing hormone (TRH) was quantified in the Mid-Hypo region. Additionally, pituitary TSHβ, follicle-stimulating hormone β (FSHβ) and LHβ were quantified for mRNA transcripts. Note that TRH is known to be produced by a group of TRH-containing neurons in the PVN located in the Mid-Hypo region, a region that showed significant elevation of TRH mRNA transcripts by d 3 of long-day photostimulation (Figure 6A). The TSHβ mRNA transcripts in the pituitary were significantly elevated by the long-day group on d 3, 7, and 28 (Figure 6B; d 28 not shown). The GnRH-1 mRNA transcripts in the SepPre/Ant-Hypo displayed no change in gene expression of GnRH-1 on d 3 and a significant decrease on d 7 in the long-day treatment group. In contrast, GnRH-1 expression was significantly elevated in the Mid-Hypo region on d 3 (Figure 6C, D). The data support a previous anatomical study showing that in the chicken, and perhaps in other avian species, the preoptic area and anterior hypothalamus has some GnRH-1 neurons; however, the ventral septum and dorsal hypothalamic area, particularly the region surrounding the nucleus of the hippocampal commissure (Figure 2B) contains a major population of GnRH-1 neurons (Kuenzel and Golden, 2006). The result is in contrast to some mammalian species where GnRH neurons are abundant in the preoptic region and in and about the arcuate nucleus in the mediobasal hypothalamus (Silverman et al., 1994). Current data also show that at the level of the pituitary, FSHβ mRNA transcripts were significantly increased on d 3, before LHβ mRNA, and continued to be significantly elevated in the 16L:8D group on d 7 and 28. The LHβ mRNA showed no change on sampling d 3 but was significantly elevated on d 7 in the long-day group (Figure 6E, F). Birds were maintained on the 2 photoperiodic treatments (8L:16D and 16L:8D) for 4 wk and testes were sampled from each group on d 3, 7, and 28. The long-day treatment resulted in a significantly elevated testes weight on d 7 and 28 compared with controls (Figure 4B).
Figure 6.
Neuroendocrine gene expression following transfer of male chicks to long-day (LD) photoperiods. A. Increased mRNA levels of thyrotropin-releasing hormone (TRH) in the middle hypothalamic (Mid-Hypo) region were shown solely on d 3 of LD birds. B. Increased thyroid stimulating hormone β (TSHβ) in anterior pituitary (Pit) was displayed on d 3 and 7 of LD photoperiods. C and D. Gene expression of gonadotropin-releasing hormone-1 (GnRH-1) levels in the septal preoptic anterior hypothalamic (SepPreAnt-Hypo) region and middle hypothalamic (Mid-Hypo) region, respectively, on d 3 and 7 after birds were transferred to LD photoperiods. E and F. Gene expression of follicle-stimulating hormone β (FSHβ) and luteinizing hormone β (LHβ), respectively, in the anterior pituitary of birds exposed to LD photoperiods on d 3 and 7 (unpublished data, S. W. Kang and W. J. Kuenzel). SD = short day. Different letters above each column in a figure are significantly different (P < 0.05).
Our data show that immature chickens can be placed in a long photoperiod and their HPG axis responds displaying significantly elevated testes growth during the very first week of light treatment. In all 3 septal-hypothalamic regions sampled on d 3, all 3 photopigments showed significantly increased mRNA transcripts in birds subjected to 16L:8D. The 3 regions (R1–3) contained 4 anatomical structures proposed to have DBP. Importantly, the Post-Hypo region (R-3) containing 2 proposed DBP structures showed significantly higher gene expression for the 2 photopigments that have been reported to be present in each site (Opn4 in locus #3 and Opn5 in locus #4, Figure 2B). Therefore it can be tentatively stated that the 4 loci shown in Figure 2B and the 3 types of photopigments/DBP (Opn4, Opn5, and VAOpn) are involved in seasonal, reproductive photo-transduction because all 3 showed significantly increased gene expression on the same day (d 3) following long-day photostimulation effective in initiating gonadal development.
Curiously, on the same sampling day (d 3), the long-day treatment group also showed significantly elevated gene expression for TRH,TSHβ,GnRH-1, and FSHβ. Hence, the activation process for both components of the PNES [(1) neural receptors (DBP) and (2) interneurons and anterior pituitary hormones (HPG axis)] occurs rapidly and documented by d 3. Therefore, a more frequent time-based sampling following onset of long photoperiods will be necessary to ascertain a possible sequence of gene activation. Many questions remain regarding the complex neuroendocrine system responsible for regulating the reproductive system of birds. Is there a dominant set of DBP in one anatomical structure that drives reproductive development? Are there different functional roles for each photopigment proposed to play a role in the photo-transduction pathway? How do the 4 anatomical sites communicate to coordinate development of this organ system? What other genes need to be examined that bring birds into reproduction? Hopefully this review will help frame future questions to clarify the stepwise function of this critically important system. Detailed knowledge of the PNES will not only serve for understanding a bird's reproductive response to a change in photoperiod, but perhaps will uncover genes, previously not examined, related to functional sustainability of their reproductive system.
Acknowledgments
We thank Kyongmin Kim for performing the electrophysiological recordings. Research was supported by the Arkansas Agricultural Experiment Station and USDA National Research Initiative Competitive Grant No. 2005-35203-15850 from Cooperative State Research, Education, and Extension Service currently known as Agriculture and Food Research Initiative from the National Institute of Food and Agriculture (W. J. K.) and National Institutes of Health grant EY017353 (Z. I. Z.).
Footnotes
Presented as part of the Symposium: Avian Reproduction: Foundational Advances and Practical Applications at the Poultry Science Association's annual meeting in Corpus Christi, Texas, July 14 to 17, 2014.
REFERENCES
- Benoit J. The role of the eye and of the hypothalamus in the photostimulation of gonads in the duck. Ann. N. Y. Acad. Sci. 1964;117:204–216. doi: 10.1111/j.1749-6632.1964.tb48175.x. [DOI] [PubMed] [Google Scholar]
- Berson D. M. Strange vision: Ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Berson D. M., Dunn F. A., Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Billings H. J., Viquie C., Karsch F. L., Goodman R. L., Connors J. M., Anderson G. M. Temporal requirements of thyroid hormones for seasonal change in LH secretion. Endocrinology. 2002;143:2618–2625. doi: 10.1210/endo.143.7.8924. [DOI] [PubMed] [Google Scholar]
- Bittman E. L., Weaver D. R. The distribution of melatonin binding sites in neuroendocrine tissues of the ewe. Biol. Reprod. 1990;43:986–993. doi: 10.1095/biolreprod43.6.986. [DOI] [PubMed] [Google Scholar]
- Chaurasia S. S., Rollag M. D., Jiang G., Hayes W. P., Haque R., Natesan A., Zatz M., Tosini G., Liu C., Korf H. W., Iuvone P. M., Provencio I. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): Differential regulation of expression in pineal and retinal cell types. J. Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- Dardente H., Klosen P., Pevet P., Masson-Pevet M. MT1 melatonin receptor mRNA expressing cells in the pars tuberalis of the European hamster: Effect of photoperiod. J. Neuroendocrinol. 2003;15:778–786. doi: 10.1046/j.1365-2826.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- Dardente H., Wyse C. A., Birnie M. J., Dupre S. M., Loudon A. S., Lincoln G. A., Hazlerigg D. G. A molecular switch for photoperiod responsiveness in mammals. Curr. Biol. 2010;20:2193–2198. doi: 10.1016/j.cub.2010.10.048. [DOI] [PubMed] [Google Scholar]
- Davies D. T., Follett B. K. The neuroendocrine control of gonadotrophin release in the Japanese quail. II. The role of the anterior hypothalamus. Proc. R. Soc. Lond. B Biol. Sci. 1975;191:303–315. doi: 10.1098/rspb.1975.0130. [DOI] [PubMed] [Google Scholar]
- Davies W. I., Turton M., Peirson S. N., Follett B. K., Halford S., Garcia-Fernandez J. M., Sharp P. J., Hankins M. W., Foster R. G. Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol. Lett. 2012;8:291–294. doi: 10.1098/rsbl.2011.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A., King V. M., Bentley G. E., Ball G. F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- El Halawani M. E., Kang S. W., Leclerc B., Kosonsiriluk S., Chaiseha Y. Dopamine-melatonin neurons in the avian hypothalamus and their role as photoperiodic clocks. Gen. Comp. Endocrinol. 2009;163:123–127. doi: 10.1016/j.ygcen.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Follett B. K., Davies D. T. Photoperiodicity and the neuroendocrine control of reproduction in birds. Symp. Zool. Soc. Lond. 1975;35:199–224. [Google Scholar]
- Foster R. G., Follett B. K., Lythgoe J. N. Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail. Nature. 1985;313:50–52. doi: 10.1038/313050a0. [DOI] [PubMed] [Google Scholar]
- Foster R. G., Grace M. S., Provencio I., Degrip W. J., Garcia-Fernandez J. M. Identification of vertebrate deep brain photoreceptors. Neurosci. Biobehav. Rev. 1994;18:541–546. doi: 10.1016/0149-7634(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Goldsmith A. R., Nicholls T. J. Thyroxine effects upon reproduction, prolactin secretion and plumage moult in intact and in thyroidectomised European Starlings Sturnus vulgaris. Ornis Scand. 1992;23:394–404. [Google Scholar]
- Gwinner E. Circannual rhythms in animals and their photoperiodic synchronization. Naturwissenschaften. 1981;68:542–551. doi: 10.1007/BF00401662. [DOI] [PubMed] [Google Scholar]
- Halford S., Pires S. S., Turton M., Zheng L., Gonzalez-Menendez I., Davies W. L., Peirson S. N., Garcia-Fernandez J. M., Hankins M. W., Foster R. G. VA opsin-based photoreceptors in the hypothalamus of birds. Curr. Biol. 2009;19:1396–1402. doi: 10.1016/j.cub.2009.06.066. [DOI] [PubMed] [Google Scholar]
- Hannibal J., Hindersson P., Knudsen S. M., Georg B., Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J. Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanon E. A., Lincoln G. A., Fustin J. M., Dardente H., Masson-Pevet M., Morgan P. J., Hazlerigg D. G. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- Harrison P. C. Extraretinal photocontrol of reproductive responses of Leghorn hens to photoperiods of different length and spectrum. Poult. Sci. 1972;51:2060–2064. doi: 10.3382/ps.0512060. [DOI] [PubMed] [Google Scholar]
- Harrison P. C., Becker W. C. Extraretinal photocontrol of oviposition in pinealectomized domestic fowl. Proc. Soc. Exp. Biol. Med. 1969;132:161–164. doi: 10.3181/00379727-132-34171. [DOI] [PubMed] [Google Scholar]
- Hattar S., Liao H. W., Takao M., Berson D. M., Yau K. W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlerigg D., Loudon A. New insights into ancient seasonal life timers. Curr. Biol. 2008;18:R795–R804. doi: 10.1016/j.cub.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Homma K., Sakakibara Y. Encephalic photoreceptors and their significance in photoperiodic control of sexual activity in Japanese quail. In: Menaker M., editor. Biochronometry. Washington, DC: Natl. Acad. Sci.; 1971. pp. 333–341. [Google Scholar]
- Kang S. W., Leclerc B., Kosonsiriluk S., Mauro L. J., Iwasawa A., El Halawani M. E. Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience. 2010;170:200–213. doi: 10.1016/j.neuroscience.2010.06.082. [DOI] [PubMed] [Google Scholar]
- Kang S. W., Leclerc B., Mauro L. J., El Halawani M. E. Serotonergic and catecholaminergic interactions with co-localised dopamine-melatonin neurones in the hypothalamus of the female turkey. J. Neuroendocrinol. 2009;21:10–19. doi: 10.1111/j.1365-2826.2008.01804.x. [DOI] [PubMed] [Google Scholar]
- Kang S. W., Thayananuphat A., Bakken T., El Halawani M. E. Dopamine-melatonin neurons in the avian hypothalamus controlling seasonal reproduction. Neuroscience. 2007;150:223–233. doi: 10.1016/j.neuroscience.2007.08.031. [DOI] [PubMed] [Google Scholar]
- King J. C., Rubin B. S. Dynamic changes in LHRH neurovascular terminals with various endocrine conditions in adults. Horm. Behav. 1994;28:349–356. doi: 10.1006/hbeh.1994.1031. [DOI] [PubMed] [Google Scholar]
- Kosonsiriluk S., Mauro L. J., Chaiworakul V., Chaiseha Y., El Halawani M. E. Photoreceptive oscillators within neurons of the premammillary nucleus (PMM) and seasonal reproduction in temperate zone birds. Gen. Comp. Endocrinol. 2013;190:149–155. doi: 10.1016/j.ygcen.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Kuenzel W. J. The search for deep encephalic photoreceptors within the avian brain, using gonadal development as a primary indicator. Poult. Sci. 1993;72:959–967. doi: 10.3382/ps.0720959. [DOI] [PubMed] [Google Scholar]
- Kuenzel W. J., Blähser S. Vasoactive intestinal polypeptide (VIP)-containing neurons: Distribution throughout the brain of the chick (Gallus domesticus) with focus upon the lateral septal organ. Cell Tissue Res. 1994;275:91–107. doi: 10.1007/BF00305378. [DOI] [PubMed] [Google Scholar]
- Kuenzel W. J. Neurobiology of molt in avian species. Poult. Sci. 2003;82:981–991. doi: 10.1093/ps/82.6.981. [DOI] [PubMed] [Google Scholar]
- Kuenzel W. J., Golden C. D. Distribution and change in number of gonadotropin-releasing hormone-1 neurons following activation of the photoneuroendocrine system in the chick, Gallus gallus. Cell Tissue Res. 2006;325:501–512. doi: 10.1007/s00441-006-0191-7. [DOI] [PubMed] [Google Scholar]
- Kuenzel W. J., Mccune S. K., Talbot R. T., Sharp P. J., Hill J. M. Sites of gene expression for vasoactive intestinal polypeptide throughout the brain of the chick (Gallus domesticus) J. Comp. Neurol. 1997;381:101–118. doi: 10.1002/(sici)1096-9861(19970428)381:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kuenzel W. J., Wideman R. F., Chapman M., Golden C., Hooge D. M. A practical method for induced moulting of caged layers that combines full access to feed and water, dietary thyroactive protein, and short day length. World's Poult. Sci. J. 2005;61:599–624. [Google Scholar]
- Lechan R. M., Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid. 2005;15:883–897. doi: 10.1089/thy.2005.15.883. [DOI] [PubMed] [Google Scholar]
- Lee S., Zhou Z. J. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron. 2006;51:787–799. doi: 10.1016/j.neuron.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman M. N., Goodman R. L., Karsch F. J., Jackson G. L., Berriman S. J., Jansen H. T. The GnRH system of seasonal breeders: Anatomy and plasticity. Brain Res. Bull. 1997;44:445–457. doi: 10.1016/s0361-9230(97)00225-6. [DOI] [PubMed] [Google Scholar]
- Leonard J. L. Dibutyryl cAMP induction of type II 5′deiodinase activity in rat brain astrocytes in culture. Biochem. Biophys. Res. Commun. 1988;151:1164–1172. doi: 10.1016/s0006-291x(88)80488-1. [DOI] [PubMed] [Google Scholar]
- Li H., Ferrari M. B., Kuenzel W. J. Light-induced reduction of cytoplasmic free calcium in neurons proposed to be encephalic photoreceptors in chick brain. Brain Res. Dev. Brain Res. 2004;153:153–161. doi: 10.1016/j.devbrainres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Li H., Kuenzel W. J. A possible neural cascade involving the photoneuroendocrine system (PNES) responsible for regulating gonadal development in an avian species, Gallus gallus. Brain Res. Bull. 2008;76:586–596. doi: 10.1016/j.brainresbull.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Malpaux B., Daveau A., Maurice-Mandon F., Duarte G., Chemineau P. Evidence that melatonin acts in the premammillary hypothalamic area to control reproduction in the ewe: Presence of binding sites and stimulation of luteinizing hormone secretion by in situ microimplant delivery. Endocrinology. 1998;139:1508–1516. doi: 10.1210/endo.139.4.5879. [DOI] [PubMed] [Google Scholar]
- Malpaux B., Migaud M., Tricoire H., Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythms. 2001;16:336–347. doi: 10.1177/074873001129002051. [DOI] [PubMed] [Google Scholar]
- McMillan J. P., Underwood J. A., Elliott J. A., Stetson M. H., Menaker M. Extraretinal light perception in the sparrow. IV. Further evidence that the eyes do not participate in photoperiodic photoreception. J. Comp. Physiol. 1975;97:205–213. [Google Scholar]
- Menaker M., Roberts R., Elliott J., Underwood H. Extraretinal light perception in the sparrow. 3. The eyes do not participate in photoperiodic photoreception. Proc. Natl. Acad. Sci. USA. 1970;67:320–325. doi: 10.1073/pnas.67.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. Y., Speh J. C., Card J. P. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J. Comp. Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Morgan P. J., Williams L. M. The pars tuberalis of the pituitary: A gateway for neuroendocrine output. Rev. Reprod. 1996;1:153–161. doi: 10.1530/ror.0.0010153. [DOI] [PubMed] [Google Scholar]
- Nakane Y., Ikegami K., Ono H., Yamamoto N., Yoshida S., Hirunagi K., Ebihara S., Kubo Y., Yoshimura T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. USA. 2010;107:15264–15268. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane Y., Shimmura T., Abe H., Yoshimura T. Intrinsic photosensitivity of a deep brain photoreceptor. Curr. Biol. 2014;24:R596–R597. doi: 10.1016/j.cub.2014.05.038. [DOI] [PubMed] [Google Scholar]
- Nakane Y., Yoshimura T. Deep brain photoreceptors and a seasonal signal transduction cascade in birds. Cell Tissue Res. 2010;342:341–344. doi: 10.1007/s00441-010-1073-6. [DOI] [PubMed] [Google Scholar]
- Nakao N., Ono H., Yamamura T., Anraku T., Takagi T., Higashi K., Yasuo S., Katou Y., Kageyama S., Uno Y., Kasukawa T., Iigo M., Sharp P. J., Iwasawa A., Suzuki Y., Sugano S., Niimi T., Mizutani M., Namikawa T., Ebihara S., Ueda H. R., Yoshimura T. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- Nakao N., Takagi T., Iigo M., Tsukamoto T., Yasuo S., Masuda T., Yanagisawa T., Ebihara S., Yoshimura T. Possible involvement of organic anion transporting polypeptide 1c1 in the photoperiodic response of gonads in birds. Endocrinology. 2006;147:1067–1073. doi: 10.1210/en.2005-1090. [DOI] [PubMed] [Google Scholar]
- Nicholls T. J., Goldsmith A. R., Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol. Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Ohuchi H., Yamashita T., Tomonari S., Fujita-Yanagibayashi S., Sakai K., Noji S., Shichida Y. A non-mammalian type opsin 5 functions dually in the photoreceptive and non-photoreceptive organs of birds. PLoS ONE. 2012;7:e31534. doi: 10.1371/journal.pone.0031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi T., Lauber J. K. Photoreception in the photosexual response of quail. I. Site of photoreceptor. Am. J. Physiol. 1973;225:155–158. doi: 10.1152/ajplegacy.1973.225.1.155. [DOI] [PubMed] [Google Scholar]
- Ono H., Nakao N., Yoshimura T. Identification of the photoperiodic signaling pathway regulating seasonal reproduction using the functional genomics approach. Gen. Comp. Endocrinol. 2009;163:2–6. doi: 10.1016/j.ygcen.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Prevot V., Bellefontaine N., Baroncini M., Sharif A., Hanchate N. K., Parkash J., Campagne C., de Seranno S. Gonadotrophin-releasing hormone nerve terminals, tanycytes and neurohaemal junction remodelling in the adult median eminence: Functional consequences for reproduction and dynamic role of vascular endothelial cells. J. Neuroendocrinol. 2010;22:639–649. doi: 10.1111/j.1365-2826.2010.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I., Jiang G., De Grip W. J., Hayes W. P., Rollag M. D. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I., Rodriguez I. R., Jiang G., Hayes W. P., Moreira E. F., Rollag M. D. A novel human opsin in the inner retina. J. Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Kumbalasiri T., Carlson S. M., Wong K. Y., Krishna V., Provencio I., Berson D. M. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Rathinam T., Kuenzel W. J. Attenuation of gonadal response to photostimulation following ablation of neurons in the lateral septal organ of chicks. Brain Res. Bull. 2005;64:455–461. doi: 10.1016/j.brainresbull.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez E. M., Blazquez J. L., Pastor F. E., Pelaez B., Pena P., Peruzzo B., Amat P. Hypothalamic tanycytes: A key component of brain-endocrine interaction. Int. Rev. Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- Saldanha C. J., Leak R. K., Silver R. Detection and transduction of daylength in birds. Psychoneuroendocrinology. 1994;19:641–656. doi: 10.1016/0306-4530(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Saldanha C. J., Silverman A. J., Silver R. Direct innervation of GnRH neurons by encephalic photoreceptors in birds. J. Biol. Rhythms. 2001;16:39–49. doi: 10.1177/074873040101600105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharrer E. Photo-neuro-endocrine systems: General concepts. Ann. N. Y. Acad. Sci. 1964;117:13–22. doi: 10.1111/j.1749-6632.1964.tb48155.x. [DOI] [PubMed] [Google Scholar]
- Sharp P. J. Photoperiodic regulation of seasonal breeding in birds. Ann. N. Y. Acad. Sci. 2005;1040:189–199. doi: 10.1196/annals.1327.024. [DOI] [PubMed] [Google Scholar]
- Silver R., Witkovsky P., Horvath P., Alones V., Barnstable C. J., Lehman M. N. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Millar R. P., King J. A., Zhuang X., Silver R. Mast cells with gonadotropin-releasing hormone-like immunoreactivity in the brain of doves. Proc. Natl. Acad. Sci. USA. 1994;91:3695–3699. doi: 10.1073/pnas.91.9.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siopes T. D., Wilson W. O. Extraocular modification of photoreception in intact and pinealectomized coturnix. Poult. Sci. 1974;53:2035–2041. doi: 10.3382/ps.0532035. [DOI] [PubMed] [Google Scholar]
- Soni B. G., Foster R. G. A novel and ancient vertebrate opsin. FEBS Lett. 1997;406:279–283. doi: 10.1016/s0014-5793(97)00287-1. [DOI] [PubMed] [Google Scholar]
- Thayananuphat A., Kang S. W., Bakken T., Millam J. R., El Halawani M. E. Rhythm-dependent light induction of the c-fos gene in the turkey hypothalamus. J. Neuroendocrinol. 2007;19:407–417. doi: 10.1111/j.1365-2826.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- Tomonari S., Takagi A., Noji S., Ohuchi H. Expression pattern of the melanopsin-like (cOpn4m) and VA opsin-like genes in the developing chicken retina and neural tissues. Gene Expr. Patterns. 2007;7:746–753. doi: 10.1016/j.modgep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Ubuka T., Bentley G. E., Tsutsui K. Neuroendocrine regulation of gonadotropin secretion in seasonally breeding birds. Front. Neurosci. 2013;7:38. doi: 10.3389/fnins.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena K., Ubuka T., Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res. 2003;312:73–79. doi: 10.1007/s00441-003-0700-x. [DOI] [PubMed] [Google Scholar]
- Van der Geyten S., Verhoelst C. H. J., Reyns G. E., Kühn E. R., Decuypere E., Darras V. M. Differential regulation of intracellular thyroid hormone availability in developing chicken brain and peripheral tissues. In: Dawson A., Sharp P. J., editors. in Functional Avian Endocrinology. New Delhi, India: Narosa Publishing House; 2005. pp. 427–436. [Google Scholar]
- Vigh B., Manzano M. J., Zadori A., Frank C. L., Lukats A., Rohlich P., Szel A., David C. Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histol. Histopathol. 2002;17:555–590. doi: 10.14670/HH-17.555. [DOI] [PubMed] [Google Scholar]
- Vigh B., Vigh-Teichmann I. Actual problems of the cerebrospinal fluid-contacting neurons. Microsc. Res. Tech. 1998;41:57–83. doi: 10.1002/(SICI)1097-0029(19980401)41:1<57::AID-JEMT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Vigh-Teichmann I., Rohlich P., Vigh B., Aros B. Comparison of the pineal complex, retina and cerebrospinal fluid contacting neurons by immunocytochemical antirhodopsin reaction. Z. Mikrosk. Anat. Forsch. 1980;94:623–640. [PubMed] [Google Scholar]
- Wada Y., Okano T., Fukada Y. Phototransduction molecules in the pigeon deep brain. J. Comp. Neurol. 2000;428:138–144. doi: 10.1002/1096-9861(20001204)428:1<138::aid-cne9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Wieselthier A. S., Van tienhoven A. The effect of thyroidectomy on testicular size and on the photorefractory period in the starling (Sturnus vulgarisL.) J. Exp. Zool. 1972;179:331–338. doi: 10.1002/jez.1401790306. [DOI] [PubMed] [Google Scholar]
- Wilson F. E. Extraocular control of photorefractoriness in American tree sparrows (Spizella arborea) Biol. Reprod. 1989;41:111–116. doi: 10.1095/biolreprod41.1.111. [DOI] [PubMed] [Google Scholar]
- Wilson F. E. Neither retinal nor pineal photoreceptors mediate photoperiodic control of seasonal reproduction in American tree sparrows (Spizella arborea) J. Exp. Zool. 1991;259:117–127. [Google Scholar]
- Wilson F. E. Photoperiodism in American tree sparrows: Role of the thyroid gland. In: Harvey S., Etches R. J., editors. in Perspectives in Avian Endocrinology. Bristol, UK: Journal of Endocrinology Ltd.; 1997. pp. 159–169. [Google Scholar]
- Wilson F. E., Reinert B. D. The photoperiodic control circuit in euthyroid American tree sparrows (Spizella arborea) is already programmed for photorefractoriness by week 4 under long days. J. Reprod. Fertil. 1995;103:279–284. doi: 10.1530/jrf.0.1030279. [DOI] [PubMed] [Google Scholar]
- Wilson F. E., Reinert B. D. Thyroid hormone acts centrally to programme photostimulated male American tree sparrows (Spizella arborea) for vernal and autumnal components of seasonality. J. Neuroendocrinol. 2000;12:87–95. doi: 10.1046/j.1365-2826.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- Yamamura T., Hirunagi K., Ebihara S., Yoshimura T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology. 2004;145:4264–4267. doi: 10.1210/en.2004-0366. [DOI] [PubMed] [Google Scholar]
- Yamamura T., Yasuo S., Hirunagi K., Ebihara S., Yoshimura T. T(3) implantation mimics photoperiodically reduced encasement of nerve terminals by glial processes in the median eminence of Japanese quail. Cell Tissue Res. 2006;324:175–179. doi: 10.1007/s00441-005-0126-8. [DOI] [PubMed] [Google Scholar]
- Yasuo S., Korf H. W. The hypophysial pars tuberalis transduces photoperiodic signals via multiple pathways and messenger molecules. Gen. Comp. Endocrinol. 2011;172:15–22. doi: 10.1016/j.ygcen.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Yasuo S., Watanabe M., Nakao N., Takagi T., Follett B. K., Ebihara S., Yoshimura T. The reciprocal switching of two thyroid hormone-activating and -inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology. 2005;146:2551–2554. doi: 10.1210/en.2005-0057. [DOI] [PubMed] [Google Scholar]
- Yasuo S., Yoshimura T. Comparative analysis of the molecular basis of photoperiodic signal transduction in vertebrates. Integr. Comp. Biol. 2009;49:507–518. doi: 10.1093/icb/icp011. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Yasuo S., Watanabe M., Iigo M., Yamamura T., Hirunagi K., Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- Zhou Z. J., Lee S. Synaptic physiology of direction selectivity in the retina. J. Physiol. 2008;586:4371–4376. doi: 10.1113/jphysiol.2008.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]