Fig. 1.
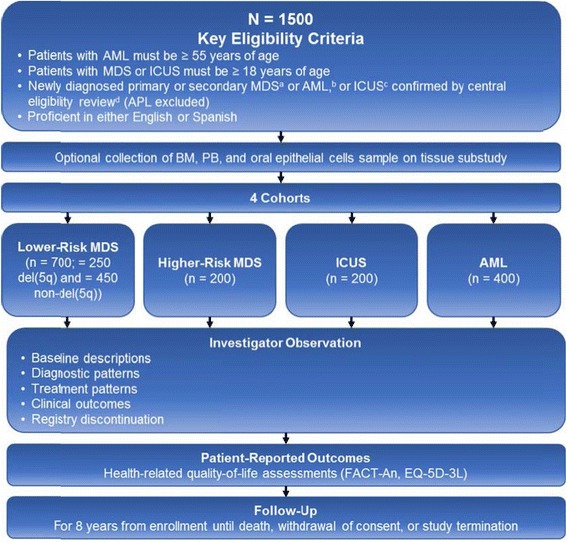
Connect MDS/AML Disease Registry study design. Overview of the study design of the disease registry from enrollment through follow-up. AML acute myeloid leukemia, APL acute promyelocytic leukemia, BM bone marrow, EQ‐5D‐3L EuroQOL. Group 5‐dimension 3‐level questionnaire, FACT‐An Functional Assessment of Cancer Therapy‐Anemia, ICUS idiopathic cytopenia of undetermined significance, MDS myelodysplastic syndromes, PB peripheral blood. aMDS diagnosis refers to the date of initial BM aspirate/biopsies for patients. bAML diagnosis refers to the date of BM aspirate/biopsies or the date of initial PB sample that led to the suspecte diagnosis. cICUS diagnosis refers to patients with ≥ 6 months’ cytopenia in ≥ 1 myeloid lineage who do not meet the criteria for diagnosis of MDS. dReview of BM aspirate/biopsies reports and cytogenetic report, PB laboratory results, or other reports that led to diagnosis of MDS or AML. Tissue samples are not reviewed; patients whose diagnosis and/or risk cannot be confirmed are deemed screen failures
