Lung cancer is a leading cause of death globally. It is also a major healthcare problem in India.[1] An online search using the words “lung cancer India” yielded the following hits on 20th February 2016. Google gave 43,80,000 results, Scholarly articles in Google were 1,77,000 and the nlm.nih.gov website in the PubMed Medline database has 2592 manuscripts Figure 1 shows the articles cited in PubMed from India on lung cancer. It is heartening to note that the healthcare community in general and oncologists in particular are steadily increasing their contribution to global medical knowledge on lung cancer. From a mere 42 publications in the year 2004 it has doubled by 2006 and then peaked at an impressive 407 (almost a ten times increase) by the year 2014 (in just a decade). Even more important is the impact this data is creating at the global level.[2,3,4] The citation index on some of the articles from India is really impressive and matches that of landmark publications. This is because some original research work included the largest series of patients of lung cancer from India, showed pharmacogenomic differences in any cancer for the first time in the world and also documented that selection of one of the options from the standard of care can be personalized to optimise outcome based on hitherto unknown criteria.[2,4,5,6] Still other publications highlight the comparison of features and outcome among patients of Asian origin (including India) and also document the survival benefit when patients with advanced lung cancer are treated by medical oncologists as opposed to other oncologist or healthcare professionals.[7,8]
Figure 1.
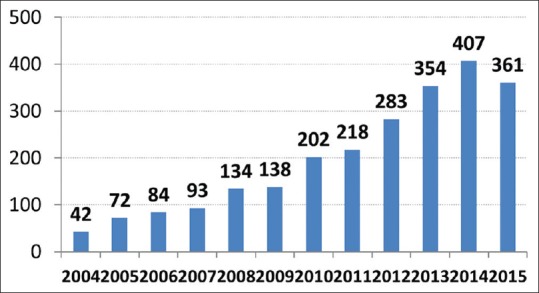
PubMed publications on lung cancer from India
We therefore congratulate Vanita et al. for nicely putting together existing Indian data on lung cancer in this issue of SAJC.[9] This editorial is to add value to their manuscript as well as discuss key additional points. Globocan estimate of lung cancer in India would indicate that incidence of lung cancer in India is 70,275 (for all ages and both genders) with an age standardized incidence rate being 6.9 per 100,000 of our population.[10] This is a gross under-estimation of the actual facts. Let us look at the data from the population based cancer registry of Indian Cancer Society from Maharashtra which covers Mumbai, Pune, Nagpur and Aurangabad.[11] This covers a population of 24,270,077 Indians (in the year 2011) - 14,275,780 from Greater Mumbai, 6,200,717 from Pune, 2,614,285 from Nagpur and Aurangabad contributing 1,179,295 residents. Table 1 shows the incidence of lung cancer in Maharashtra as documented in 2011 by these four population based cancer registries. They collectively recorded 3170 new cases. By extrapolating this data to the 1.16 billion Indians, it shows that the actual new cases across India were 156,736 new cases, more than double of what is estimated by Globocan! Thi1 fact needs to be given priority when planning for the requirement of infrastructure, human resources as well as resource allocation for our country.
Table 1.
Incidence of lung cancer in Maharashtra in 2011-data from Indian Cancer Society’s population based cancer registries
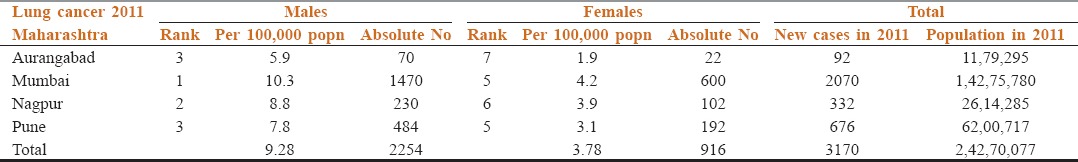
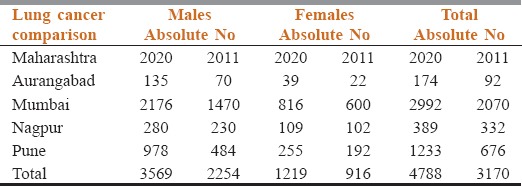
Table 2 shows the projected change in incidence of lung cancer expected in Maharashtra by 2020 – which is only 4 years away. In the four cities of Maharashtra (Mumbai, Pune, Nagpur and Aurangabad) the absolute numbers of newly diagnosed lung cancer patient will increase from 3170 to 4788 (more than 50% increase). At a national level this would translate into 235,104 new patients. With 90% of these presenting in an advanced inoperable stage, the future looks extremely challenging.
Table 2.
projected increase in incidence of lung cancer by 2020 in Maharashtra
Hence we need to take advantage of technology that has the potential to improve outcome in such patients using the personalized medicine approach. In this editorial, we shall discuss only three which are most promising and immediately applicable.
Molecular Oncology
Over the last 10 years the survival in lung cancer has increased from a median overall survival of 11 months to an overall 5-year survival rate of 17.8%.[1] This benefit is mainly due to the availability of targeted therapy drugs and the appropriate selection of the patients – in other words precision oncology and personalized medicine. To a large extend this is possible only due to molecular oncology. Currently molecular testing in lung cancer has become mandatory, is part of all management guidelines globally and is easily available in India as well.[6] Noronha et al. have elegantly summarized the current Indian data on this.[9,12,13] Others have also used this concept to improve patient outcome.[14] For this editorial, we would like to stress on two aspects. The first one is that besides indentifying driver mutations, it is equally important to ascertain how to select the most effective and least toxic chemotherapy combination for patients with advanced lung cancer (even today, about 75% of lung cancer patients will still require chemotherapy at some time during their illness). Testing for SNPs and understanding their pharmacokinetic/pharmacodynamic implications requires careful attention. This will not only help us select the right combination (from among the standard of care options) but also fine tune their dosage as well as supportive care required. This can optimise personal approach for each patient with a focus on QoL as well as response rates. The second aspect is ensuring quality of the reports from the labs doing molecular testing – an important unmet need at present. Hence we are in the process of setting up a nationwide external quality assurance program (EQAP) for molecular testing in India. We are starting with EGFR testing for lung cancer this year and will expand our services quickly. We encourage all stake holders to insist on molecular laboratories participating in our pan India EQAP so that we have the confidence that clinical treatment decisions are made on the basis of sound and reliable molecular laboratory reports.
Immuno Oncology
Application of immuno oncology in lung cancer is nothing short of a revolution. It is a form of personalized medicine that offers a groundbreaking tool in the fight against lung cancer. Immune checkpoint inhibitors now offer a novel way of tackling cancer and improve outcome. ASCO has therefore labelled cancer immunotherapy as Advance of the year 2015.[15] In fact, ASCO University has even developed a six module training program for immune oncology. We invite you to read more about this in the forthcoming issue of International Journal of Molecular and Immuno Oncology which is being launched by us in the third quarter of 2016 (www.ijmio.com). Nivolomab (Opdivo) was approved in October 2015 for lung cancer. It is both a monoclonal antibody and a “checkpoint inhibitor” that attacks programmed death 1 pathway. Pembrolizumab (Keytruda) was also approved in the same month but requires a “companion diagnostic” test. Other promising immunotherapy approaches being studied include Yervoy (ipilumimab), Atezolizumab (MPDL3280A) and Durvalumab (MEDI4736).
Artificial Intelligence
Imaging Computation and algorithms that allows faster, more accurate and consistent evaluation of lesions is not only progressing exponentially but is crucial in lung cancer for two reasons. First it will be able to distinguish whether a solitary pulmonary nodule is benign or malignant.[16,17] And secondly it has the potential to allow early diagnosis, which will allow us to diagnose lung cancer when it is operative with curative intent.[17,18] At the forefront of this approach are Tomas Vykruta and Joe Bertolami from the Microsoft Kinect project. We are in discussion with them to devise computer algorithm to distinguish pulmonary tuberculosis from lung cancer with a high degree of accuracy.
References
- 1.Parikh PM, editor. Lung Cancer Monograph 2012. ICP, API. 2012 [Google Scholar]
- 2.Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 3.Parikh PM, Vaid A, Advani SH, Digumarti R, Madhavan J, Nag S, et al. Randomized, double-blind, placebo-controlled phase II study of single-agent oral talactoferrin in patients with locally advanced or metastatic non-small-cell lung cancer that progressed after chemotherapy. J Clin Oncol. 2011;29:4129–36. doi: 10.1200/JCO.2010.34.4127. [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–42. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 6.Parikh P, Puri T. Personalized medicine: Lung cancer leads the way. Indian J Cancer. 2013;50:77–9. doi: 10.4103/0019-509X.117005. [DOI] [PubMed] [Google Scholar]
- 7.Chang A, Parikh P, Thongprasert S, Tan EH, Perng RP, Ganzon D, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: Subset analysis from the ISEL study. J Thorac Oncol. 2006;1:847–55. [PubMed] [Google Scholar]
- 8.Parikh PM. Treatment of lung cancer in the elderly. Inj J Med Peadiatr Oncol. 1999;2:140–7. [Google Scholar]
- 9.Vanita N, Pinnati R, Joshi A, Patil VM, Prabhash K. Lung cancer in the Indian subcontinent. SAJC. 2016;5:95–103. doi: 10.4103/2278-330X.187571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [Last accessed on 2016 Feb 20]. Available from: http://www.globocan.iarc.fr/Pages/fact_sheets_cancer.aspx .
- 11. [Last accessed on 2016 Feb 20]. Available from: http://www.indiancancersociety.org/cancer-registry/cancer-registry.aspx .
- 12.Chougule A, Prabhash K, Noronha V, Joshi A, Thavamani A, Chandrani P, et al. Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS One. 2013;8:e76164. doi: 10.1371/journal.pone.0076164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noronha V, Joshi A, Gokarn A, Sharma V, Patil V, Janu A, et al. The importance of brain metastasis in EGFR mutation positive NSCLC patients. Chemother Res Pract 2014. 2014:856156. doi: 10.1155/2014/856156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veldore VH, Patil S, Prabhudesai S, Satheesh CT, Shashidhara HP, Krishnamoorthy N, et al. Targeted therapy management in NSCLC patients using cytology: Experience from a tertiary care cancer center. Mol Diagn Ther. 2016;20:119–23. doi: 10.1007/s40291-015-0180-1. [DOI] [PubMed] [Google Scholar]
- 15. [Last accessed on 2016 Feb 20]. Available from: http://www.cancerprogress.net/cca/advance-year-cancer-immunotherapy .
- 16.Juntu J, Sijbers J, De Backer S, Rajan J, Van Dyck D. Machine learning study of several classifiers trained with texture analysis features to differentiate benign from malignant soft-tissue tumors in T1-MRI images. J Magn Reson Imaging. 2010;31:680–9. doi: 10.1002/jmri.22095. [DOI] [PubMed] [Google Scholar]
- 17.Walia S, Sharma S, Markand Kulurkar P, Patial V, Acharya A. A bimodal molecular imaging probe based on chitosan encapsulated magneto-fluorescent nanocomposite offers biocompatibility, visualization of specific cancer cells in vitro and lung tissues in vivo. Int J Pharm. 2016;498:110–8. doi: 10.1016/j.ijpharm.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Sampedro F, Escalera S, Domenech A, Carrio I. A computational framework for cancer response assessment based on oncological PET-CT scans. Comput Biol Med. 2014;55:92–9. doi: 10.1016/j.compbiomed.2014.10.014. [DOI] [PubMed] [Google Scholar]


