Abstract
Background:
Inflammatory bowel disease (IBD) is an intestinal chronic inflammatory condition and includes Crohn's disease (CD) and ulcerative colitis (UC). It has been proposed that Vitamin D supplementation may have a beneficial role in IBD.
Aim:
To characterize the effects of Vitamin D on cathelicidin (hCAP/LL37) gene expression, ESR, and serum hs-CRP levels.
Materials and Methods:
Ninety UC patients on remission were randomized to receive 300,000 IU intramuscular Vitamin D or 1 mL normal saline as placebo, respectively. Before and 90 days after intervention, serum levels of 25 (OH)-Vitamin D3, PTH, Calcium, ESR, and hs-CRP were measured. Cathelicidin gene expression was also quantified using qRT-PCR.
Results:
Baseline serum 25-OH-Vitamin D3 levels were not different between the two groups and after intervention, increased only in Vitamin D group (P < 0.001). Hs-CRP levels were lower in Vitamin D group after intervention (Before: 3.43 ± 3.47 vs 3.86 ± 3.55 mg/L, P = 0.56; after: 2.31 ± 2.25 vs 3.90 ± 3.97 mg/L, P = 0.023). ESR decreased significantly in Vitamin D group (Before: 12.4 ± 6.1 vs 12.1 ± 5.3 mm/h, P = 0.77; after: 6.7 ± 4.5 vs 11.4 ± 5.5 mm/h, P < 0.001). The mean fold change in hCAP18 gene expression in Vitamin D group was significantly higher than placebo group. (Mean ± SD: 3.13 ± 2.56 vs 1.09 ± 0.56; median ± interquartile range: 2.17 ± 3.81 vs 0.87 ± 0.53, P < 0.001).
Conclusion:
Decreases in ESR and hs-CRP levels and increase in LL37 gene expression support the hypothesis that Vitamin D supplementation may have a beneficial role in UC patients.
Key Words: Cathelicidin (hCAP18/LL37), ESR, hs-CRP, inflammatory bowel disease, ulcerative colitis, Vitamin D
Inflammatory bowel disease (IBD) is an intestinal chronic inflammatory condition with remission–relapse periods, and includes two forms, Crohn's disease (CD) and ulcerative colitis (UC). IBD is associated with abnormal immune responses[1] and to date there is no cure for IBD, whereas the goal of medical therapy is to achieve and maintain remission.[2]
Antimicrobial peptides (AMPs) are endogenous antibiotics with antimicrobial and anti-inflammatory activities. AMPs are expressed in intestinal, epithelial, Paneth, and immune cells.[3]
Cathelicidin antimicrobial peptides (CAMPs) are a family of AMPs expressed in immune cells, intestinal epithelial cells, and skin.[4] LL37 is the only cathelicidin described so far in humans. It is synthesized as a precursor entitled human cationic antimicrobial protein 18 (hCAP18). The expression of LL37 is stimulated in colon epithelial cells by bacterial components such as butyrate.[5]
It has been shown that in the colon of patients with UC the expression of LL37 increases.[6] Symptoms of acute colitis in mCAMP knockout mouse are more severe.[7]
Enhancing immune system by increasing the induction expression of AMPs in the body has been proposed. 1,25 (OH) 2-D3 is known to induce the expression of cathelicidin;[8] For example, calcipotriol ointment (Vitamin D analogue) increases the production of cathelicidin in the skin of patients with psoriasis.[9]
Two sources of Vitamin D are diet and endogenous production by skin. Vitamin D synthesizes in the skin under the effect of solar ultraviolet (UVB) radiation. Fish, liver, and fortified products are main dietary sources of Vitamin D intakes.[10] 25OH-D3 has a half-life of about 15 days,[11] and it is typically used as an indicator of Vitamin D status. Vitamin D sufficiency is defined as serum 25 (OH)-D3 levels of 30–50 ng/mL and Vitamin D toxicity is observed at serum 25 (OH)-D3 levels more than 150 ng/mL.[12]
The abilities of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) as indicators of inflammation to identify UC patients that are likely to undergo relapses have also been examined and in several studies increased levels of CRP and/or ESR have been associated with relapse.[13] It has been shown that 1,25 (OH) 2D3 inhibits excessive inflammation[14] and suppresses the production of pro-inflammatory cytokines by dendritic cells (DCs) and innate immune cells.[15]
The physiological doses of Vitamin D deficiency do not guarantee its immunomodulatory benefits. It is more likely that the pharmacological doses of Vitamin D are necessary to achieve immunomodulatory effects.[16]
The aim of this study is to evaluate the effect of single high-dose intramuscular Vitamin D injection on LL37 gene expression, high-sensitivity C-reactive protein (hs-CRP), and ESR as indicators of inflammation in previously diagnosed UC patients.
MATERIALS AND METHODS
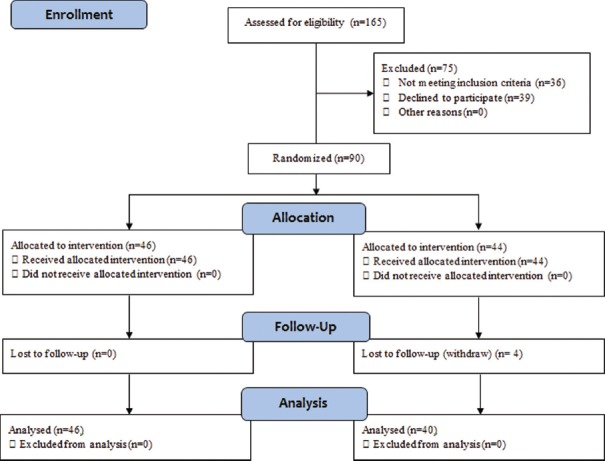
This study is a double-blind randomized controlled trial with parallel design and 1:1 ratio of two groups. The calculated total sample size with 80% power using a two-sided test at the 5% significance level was 86 persons and after considering 5% loss to follow-up 90 persons were calculated.[17,18] One hundred and sixty-five patients were invited for assessing eligibility to participate in the trial between December 2014 and January 2015; of whom 39 persons declined to participate and 36 persons did not meet the criteria. Ninety persons were randomized manually using Stratified Blocked Randomization method. Strata were based on age (≤35 years/>35 years) and BMI (≤25/>25 kg/m2) with blocks of 4. The CONSORT flow diagram of trial is presented in Figure 1. This clinical trial was registered at the Iranian registry of Clinical trials (IRCT): IRCT2014062318207N1. The study was approved by Tehran University of Medical Sciences Ethics Committee, and a written informed consent was obtained from all participants before enrolment.
Figure 1.
Consort diagram of intervention
All patients were previously diagnosed by standard clinical, radiographic, endoscopic, and histopathologic criteria and were not at a relapse phase. Exclusion criteria were age less than 18 and more than 50 years; body mass index (BMI) lower than 18.5 or higher than 30 kg/m2; anti-TNF-alpha therapy; taking any form of Vitamin D3 supplement in the 3 months preceding the study; history of hyperparathyroidism, nephrolithiasis, malignancy or renal or hepatic failure; and pregnancy and breastfeeding.
The participants were assigned to receive one muscular injection of 1 mL 300,000 IU Vitamin D3 or 1 mL normal saline as placebo. Intervention staff who delivered the intervention did not take outcome measurements. Investigators and participants were kept masked to allocation.
Before intervention and after 90 days follow-up, participants completed a clinical assessment including measurement of height, weight, blood pressure, heart rate, body temperature, and a 3-day 24 h food recall, including weekend, were taken. Blood samples were taken for RNA isolation and serum separation.
Statistical analyses were performed using SPSS 20.0 (IBM SPSS Inc., Chicago, IL, United States) and STATA version 12 (Stata Statistical Software, College Station, TX, USA). The results are expressed as mean ± standard deviation (SD) or number (%). Data with skewed distributions are expressed as medians and interquartile ranges.
Experiments
Blood samples were obtained in plain tubes, and serums were separated by centrifuging at 2500 rpm for 10 min after 30 min at 37°C, and stored at −80°C. Circulating levels of 25 (OH) D3 were determined using commercial ELISA kit (Calbiotech, CA, USA) according to manufacturer's instructions/procedure. Serum hs-CRP levels were also measured using ELISA kit (Monobind, CA, USA), and PTH serum levels were assessed using ELISA kit (Euroimmun, Luebeck, Germany).
LL-37 gene expression was determined by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis using the succinate dehydrogenase complex subunit A (SDHA) gene[19] as a reliable internal control. Total RNA was immediately isolated from 1 mL whole blood using the QIAamp RNA Blood Mini Kit (Qiagen, USA) according to manufacturer's procedure, and samples were treated with DNase 1 to remove the remaining genomic DNA. Optical densities of total RNA were measured at 280, 260, and 230 nm for quality and quantity. RNA samples were stored at −80°C.
Reverse transcription of 1 µg total RNA was performed using GeneAll HyperScript™ Reverse Transcriptase (GeneAll, South Korea) with oligo dT and random hexamer primers in 20 μL reaction volume as described by the manufacturer.
Immediately after cDNA synthesis, duplicate real-time qualitative PCR was performed using The LightCycler® 2.0 Instrument (Roche, Germany). Each reaction mixture contained 10 μL of 2 × SYBR Green Master Mix (Amplicon), 1 µL of each Forward and Reverse primer (10 ng/μL), and 1 μL of cDNA (equal to 50 ng RNA) and PCR-Grade water in a final volume of 20 μL. Controls without reverse transcriptase and controls without template (NTC) were included in each experiment as quality control. A standard curve of four serial dilutions of cDNAs (50 ng to 50 pg) was obtained; PCR efficiencies were above 95%.
The LL-37/hCAP18 (ENSG00000164047; Chromosome 3:48,223,347-48,225,491; 2,145 bp) and SDHA (ENSG00000073578; Chromosome 5:218,241-256,700; 38,460 bp) primers were as follows: LL-37, Forward: 5′-GCTGGGTGATTTCTTCCGGA-3′; Reverse: 5′-CCTGGGTACAAGATTCCGCA-3′ and SDHA, forward: 5′-TGGGAACAAGAGGGCATCTG-3′; Reverse: 5′-CCACCACTGCATCAAATTCATG-3′.[20]
The amplification profile was 10 min initial denaturation at 95°C followed by 15 s of denaturation at 95°C, 20 s of annealing at 56°C, and 20 s extensions at 72°C, for a total of 40 cycles. For Melt Curve analysis, the instrument was set as follows: 15 s at 95°C, 60 s at 60°C, and 95°C, 0.1°C/s slope.
Fold changes in LL-37/hCAP18 mRNA expression normalized to SDHA and relative to baseline were determined for each participant using the 2−(ΔΔCt) or 2−ΔΔCq method[21] using formulated Excel (Microsoft Office 2010), where: ΔΔCq = (CqLL37 After− CqSDHA After) − (CqLL37 Before− CqSDHA Before).
RESULTS
Baseline comparisons were done for all the 90 participants. Four patients in placebo group withdrew from the study; there were no significant differences between these four patients and others regarding baseline characteristics indicated in Table 1. At the end of the study, data of 86 participants (40 from the placebo and 46 from Vitamin D groups) were analyzed. Intention-to-treat (ITT) analyses were also performed, but it did not change the results regarding statistical significance. Clinical and demographic characteristics of participants were comparable for intervention versus control group at baseline [Table 1].
Table 1.
Characteristics of patients before and after the intervention
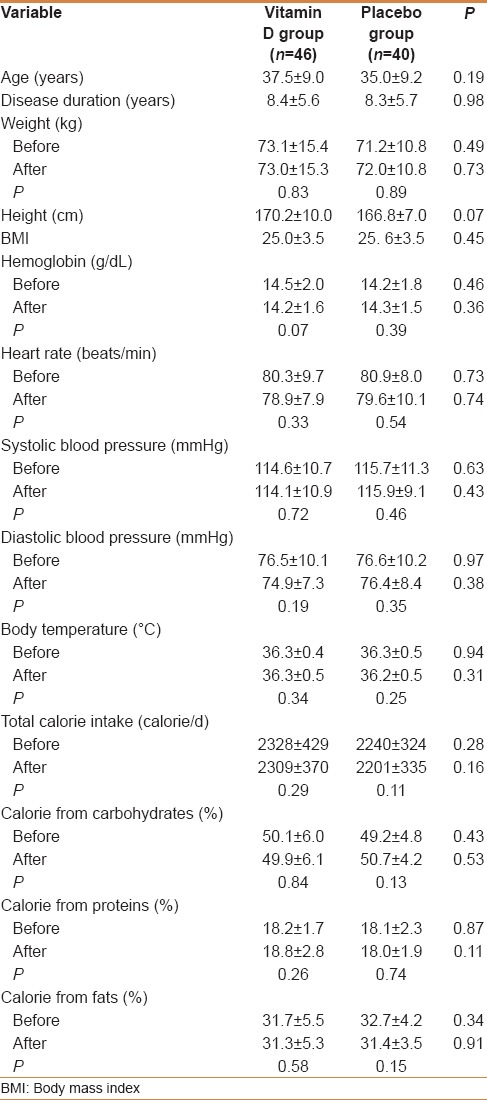
There were also no differences between groups regarding gender and type of drugs [Table 2]. The drug regimen of all patients remained unchanged during the study.
Table 2.
Participant's gender and type of drugs
Baseline serum 25-OH-vitamin D3 levels were not different between the two groups (P = 0.82) and after intervention it increased in Vitamin D group (P < 0.001), but remained unchanged in the placebo group compared with baselines (P = 0.13) [Table 3].
Table 3.
Serum levels of 25-OH-vitamin D3, calcium, and parathyroid hormone before and after intervention
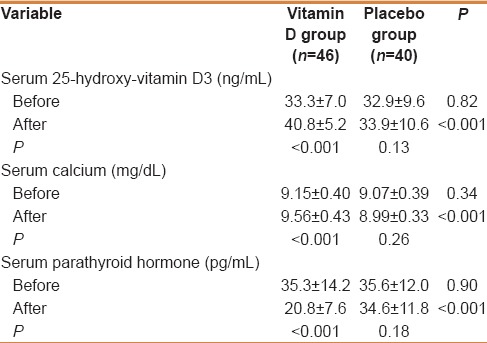
There were significant increases in calcium levels in Vitamin D group but not in placebo group. At the end of the study, both Vitamin D and calcium levels were in normal intoxicant ranges in Vitamin D group (25-OH-Vitamin D3: 29.6-51.4 ng/mL and Calcium: 8.6-10.4 mg/dL). PTH levels decreased by 41% in Vitamin D group but did not change in placebo group [Table 3].
Hs-CRP levels were lower in Vitamin D group after intervention (Before: 3.43 ± 3.47 vs 3.86 ± 3.55 mg/L, P = 0.56; after: 2.31 ± 2.25 vs 3.90 ± 3.97 mg/L, P = 0.023) [Figure 2]. The mean hs-CRP changes in Vitamin D and placebo groups was − 1.12 ± 3.50 and + 0.17 ± 1.92 mg/L, respectively (P = 0.036).
Figure 2.
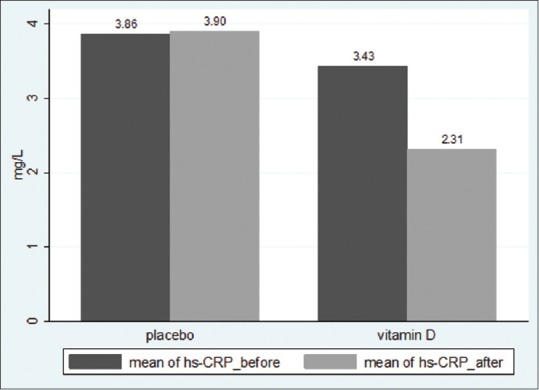
Mean hs-CRP levels before and after intervention in Vitamin D and Placebo groups (P = 0.036)
ESR decreased by 46% compared with baseline in Vitamin D group, whereas there was no significant change in the placebo group (Before: 12.4 ± 6.1 vs 12.1 ± 5.3 mm/h, P = 0.77; after: 6.7 ± 4.5 vs 11.4 ± 5.5 mm/h, P < 0.001) [Figure 3]. The mean ESR changes in Vitamin D and placebo groups were − 5.76 ± 3.77 and − 0.13 ± 2.55 mm/h, respectively (P < 0.001).
Figure 3.
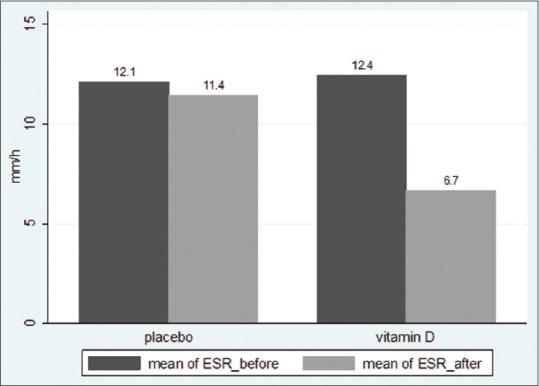
Mean ESR levels before and after intervention in Vitamin D and Placebo groups (P < 0.001)
Real-time quantitative PCR experiment showed that the mean change fold in hCAP-18 gene expression in Vitamin D group was significantly higher than that observed in the placebo group. (Mean ± SD: 3.13 ± 2.56 vs 1.09 ± 0.56; Median ± interquartile range: 2.17 ± 3.81 vs 0.87 ± 0.53, P < 0.001) [Figure 4].
Figure 4.
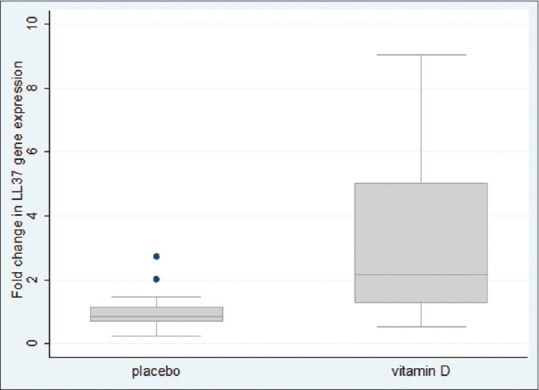
Box and whisker plots show the fold change in gene expression of LL37/hCAP18 normalized to SDHA and calibrated for baselines in 86 UC patients 90 days after receiving 300,000 IU vitamin D or placebo, respectively (P < 0.001)
DISCUSSION
In this study, we evaluated the effect of single high-dose (300,000 IU) Vitamin D injection on hCAP18/LL37 gene expression, hs-CRP levels, and ESR in UC patients with mild to moderate disease condition. High-dose vitamin D was effective in elevating serum 25 (OH) D levels. We found that vitamin D leads to increase in cathelicidin gene expression and decreases in hs-CRP and ESR levels as indicators of inflammation.
There are increasing evidences linking Vitamin D deficiency and autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, diabetes mellitus, and inflammatory bowel disease.[22]
There is an increasing interest in Vitamin D beyond its traditional role in calcium homeostasis including anti-inflammatory and immune-modulating effects in immune-mediated diseases such as multiple sclerosis and inflammatory bowel diseases. The association of northern latitudes with higher prevalence of autoimmune diseases led to the implication of Vitamin D in the pathogenesis of these diseases.[16] For example, people who live near the equator are at a low risk of developing IBD.[23]
Vitamin D deficiency is associated with increased autoimmune diseases and an increased susceptibility to infection. Vitamin D receptors (VDR) are expressed in immune cells and these cells convert Vitamin D to its active metabolite.[22] So the beneficial effects of vitamin D supplementation in autoimmune disease may have the effects beyond bone and calcium homeostasis.[22]
The most commonly used proxy markers of systemic inflammation in IBD are CRP and ESR, and patients with higher levels of these markers are more susceptible to relapse.[24] CRP and ESR also have high correlation with clinical and endoscopic findings in both CD and UC.[13,25] In addition elevated levels of CRP or ESR are associated with increased risk of colorectal cancer in patients with IBD.[26,27,28] High CRP levels at remission are also associated with increased relapse risk after therapy withdrawal.[29] In a study IBD patients with higher ESR had significantly lower 25 (OH) D than controls.[30] In our study there were 32% decreases in hs-CRP levels and 46% decrease in ESR compared to baselines in Vitamin D receiving group, illustrating the remarkable anti-inflammatory benefit of Vitamin D in patients with UC.
Vitamin D plays an important role in the innate antimicrobial response. Important insight into the mechanism of production of antimicrobial peptides came with the discovery of the VDR response elements (VDREs) in the promoter of CAMP gene, and the finding that the conversion of Vitamin D to its active metabolite occurs in keratinocytes and monocytes under the control of Toll-like receptor 2 (TLR).[31] With infection or wounding, activation of TLR causes increased expression of both the 1-α-hydroxylase (CYP27B1) and VDR.[32,33] CYP27B1 converts 25-hydroxyvitamin D to the 1,25-dihydroxyvitamin D, and this leads to binding of the 1,25 (OH) 2D3-VDR-RXR heterodimer to the VDREs of the LL37 gene. Expression of cathelicidin is dependent on sufficient Vitamin D levels.[32]
Cathelicidin expression is increased in inflamed and non-inflamed intestinal mucosa of UC patients.[6] When cathelicidin was administered into the rectum of DSS-induced enteritis mice, mucin-4 gene expression was increased and colonic mucosal thickness was restored, and inducted colitis was cured.[34] It has been also shown that endogenous LL37 has an anti-inflammatory role in the development of DSS-induced colitis in mice.[7]
In one study six months of cholecalciferol/calcium supplementation in Vitamin D deficient young healthy females had no significant alteration in expression of LL37.[35] Although in another study, 4000 IU/d oral Vitamin D supplementation in Atopic Dermatitis lesional skin patients leads to increase in LL37 gene expression from a median of 3.53 relative copy units before supplementation to a median of 23.91 relative copy units 21 days after supplementation. Normal skin showed a more modest increase, and AD nonlesional skin mildly increased.[36] In our study Vitamin D enhanced cathelicidin gene expression by an average 3.13-fold (median: 2.17 vs 0.87) that endorses studies done on AD patients.
A recent meta-analysis study showed that IBD is significantly associated with higher odds of Vitamin D deficiency.[37] In one study, Vitamin D supplementation reduced the severity of colitis in wild-type mice.[38] Also in another study Vitamin D significantly reduced clinical severity in IBD-induced mice model, and its combination with dexamethasone demonstrated more reduction of IBD severity.[39] There are many animal studies on the effects of Vitamin D in IBD; however to date, there is only one published clinical trial in human IBD patients in which eight patients in stable clinical remission were administered a high dose (250,000 or 500,000 IU) of Vitamin D intramuscularly within 2 weeks following infliximab infusion and 8 weeks after Vitamin D supplementation were compared with 12 patients who were not Vitamin D deficient and did not receive supplement. There was no report regarding ESR and CRP levels and LL37 gene expression.[40]
The analysis of basic characteristics shows that there are no differences between groups before and after studies specially in calorie intakes. In addition heart rate, body temperature, blood pressure, and hemoglobin levels did not change over intervention that may be due to the fact that all the patients were in remission phase of disease. Further interventions in relapse phase patients are needed to evaluate the effect of Vitamin D on these variables.
To date there is no consensus regarding the optimal levels of serum Vitamin D.[41] Vitamin D toxicity has been set to occur at serum 25 (OH) D concentrations higher than 150 ng/mL.[12] In a small study of pediatric IBD patients, 800,000 IU single doses were safe and effective.[42] In our study the mean serum 25-OH-D increase was 7.5 ng/mL in Vitamin D group 3 months after intervention, and the range of observations remained in safe level (29.6 to 51.4 ng/mL); and also serum calcium levels were in normal and safe ranges (8.6–10.4 mg/dL) and no subject complained of any adverse event.
By lowering the inflammation in IBD patients, induction or maintenance of remission may be achieved. In one study 1200 IU/d Vitamin D supplementation reduced CD relapse rate from 29% to 13% (P < 0.06). Besides these, recent studies have shown an inverse correlation between serum Vitamin D levels and the incidence of polyps and adenomas in the colon[43,44] that are common complications of UC. Vitamin D has additional benefits to UC patients including bone health and treating depressive symptoms. So Vitamin D desires to be considered by clinicians as an adjuvant treatment in UC patients. However, further studies to assess the effect of Vitamin D in UC patients with active disease status are needed.
Financial support and sponsorship
This work was supported by Tehran University of Medical Sciences and Health Services grant (No. 27089).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Carter MJ, Lobo AJ, Travis SP. IBD Section, British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–94. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Ho S, Pothoulakis C, Koon HW. Antimicrobial peptides and colitis. Curr Pharm Des. 2013;19:40–7. doi: 10.2174/13816128130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals R, Wilson JM. Cathelicidins – A family of multifunctional antimicrobial peptides. Cell Mol Life Sci. 2003;60:711–20. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, et al. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol. 2004;41:847–54. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Schauber J, Rieger D, Weiler F, Wehkamp J, Eck M, Fellermann K, et al. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2006;18:615–21. doi: 10.1097/00042737-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Koon HW, Shih DQ, Chen J, Bakirtzi K, Hing TC, Law I, et al. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology. 2011;141:185–63.e1-3. doi: 10.1053/j.gastro.2011.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–31. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Törmä H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–2. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Jones G. Pharmacokinetics of Vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–6S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–26.e2. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–70. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 15.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. J Mol Med (Berl) 2010;88:441–50. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raman M, Milestone AN, Walters JR, Hart AL, Ghosh S. Vitamin D and gastrointestinal diseases: Inflammatory bowel disease and colorectal cancer. Therap Adv Gastroenterol. 2011;4:49–62. doi: 10.1177/1756283X10377820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owczarek D, Cibor D, Mach T. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), arginine, and 8-iso-prostaglandin F2alpha (8-iso-PGF2alpha) level in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:52–7. doi: 10.1002/ibd.20994. [DOI] [PubMed] [Google Scholar]
- 18.Sakpal TV. Sample size estimation in clinical trial. Perspect Clin Res. 2010;1:67–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Ledderose C, Heyn J, Limbeck E, Kreth S. Selection of reliable reference genes for quantitative real-time PCR in human T cells and neutrophils. BMC Res Notes. 2011;4:427. doi: 10.1186/1756-0500-4-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira F, Barbáchano A, Singh PK, Campbell MJ, Muñoz A, Larriba MJ. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle. 2012;11:1081–9. doi: 10.4161/cc.11.6.19508. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–6. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carr I, Mayberry JF. The effects of migration on ulcerative colitis: A three-year prospective study among Europeans and first-and second- generation South Asians in Leicester (1991-1994) Am J Gastroenterol. 1999;94:2918–22. doi: 10.1111/j.1572-0241.1999.01438.x. [DOI] [PubMed] [Google Scholar]
- 24.Papi C, Festa V, Leandro G, Moretti A, Tanga M, Koch M, et al. Long-term outcome of Crohn's disease following corticosteroid-induced remission. Am J Gastroenterol. 2007;102:814–9. doi: 10.1111/j.1572-0241.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 25.Solem CA, Loftus EV, Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–12. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 26.Ananthakrishnan AN, Cheng SC, Cai T, Cagan A, Gainer VS, Szolovits P, et al. Serum inflammatory markers and risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:1342–8.e1. doi: 10.1016/j.cgh.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aleksandrova K, Jenab M, Boeing H, Jansen E, Bueno-de-Mesquita HB, Rinaldi S, et al. Circulating C-reactive protein concentrations and risks of colon and rectal cancer: A nested case-control study within the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172:407–18. doi: 10.1093/aje/kwq135. [DOI] [PubMed] [Google Scholar]
- 28.Burstein E, Fearon ER. Colitis and cancer: A tale of inflammatory cells and their cytokines. J Clin Invest. 2008;118:464–7. doi: 10.1172/JCI34831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oussalah A, Chevaux JB, Fay R, Sandborn WJ, Bigard MA, Peyrin-Biroulet L. Predictors of infliximab failure after azathioprine withdrawal in Crohn's disease treated with combination therapy. Am J Gastroenterol. 2010;105:1142–9. doi: 10.1038/ajg.2010.158. [DOI] [PubMed] [Google Scholar]
- 30.Veit LE, Maranda L, Fong J, Nwosu BU. The Vitamin D status in inflammatory bowel disease. PLoS One. 2014;9:e101583. doi: 10.1371/journal.pone.0101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a Vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a Vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 33.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 34.Tai EK, Wong HP, Lam EK, Wu WK, Yu L, Koo MW, et al. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J Cell Biochem. 2008;104:251–8. doi: 10.1002/jcb.21615. [DOI] [PubMed] [Google Scholar]
- 35.Das M, Tomar N, Sreenivas V, Gupta N, Goswami R. Effect of Vitamin D supplementation on cathelicidin, IFN-γ, IL-4 and Th1/Th2 transcription factors in young healthy females. Eur J Clin Nutr. 2014;68:338–43. doi: 10.1038/ejcn.2013.268. [DOI] [PubMed] [Google Scholar]
- 36.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral Vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–31. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and Vitamin D deficiency: A systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2708–17. doi: 10.1097/MIB.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Froicu M, Cantorna MT. Vitamin D and the Vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 40.Reich K, Madsen K, Foshaug RR, Fedorak RN, Kroeker KI. 184 high dose Vitamin D supplementation stimulates innate cytokine responses in patients with inflammatory bowel disease on infliximab. Gastroenterology. 2014;146(5):48. [Abstract] [Google Scholar]
- 41.Narula N, Marshall JK. Management of inflammatory bowel disease with vitamin D: Beyond bone health. J Crohns Colitis. 2012;6:397–404. doi: 10.1016/j.crohns.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Day AS, Lemberg DA, Messenger R, Woodhead HJ. High dose Vitamin D therapy in paediatric IBD. Gastroenterology. 2011;140(5):512. [Abstract] [Google Scholar]
- 43.Moon M, Song H, Hong HJ, Nam DW, Cha MY, Oh MS, et al. Vitamin D-binding protein interacts with Aß and suppresses Aß-mediated pathology. Cell Death Differ. 2013;20:630–8. doi: 10.1038/cdd.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–24. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]