ABSTRACT
DNA replication is constantly challenged by both endogenous and exogenous sources of replication stress. SMARCAL1, an SNF2 family DNA translocase, functions in the DNA damage response to address these obstacles and promote the completion of replication. Most studies examining the function of SMARCAL1 and related enzymes have relied on the addition of exogenous genotoxic agents, but SMARCAL1 is needed even in the absence of these drugs to maintain genome stability during DNA replication. We recently determined that SMARCAL1 functions to limit DNA damage during replication of difficult-to-replicate telomere sequences. SMARCAL1-deficient cells display several markers of telomere instability including extrachromosomal telomere circles and co-localization with DNA damage markers. Furthermore, cells lacking the highly related proteins ZRANB3 and HLTF do not exhibit similar problems suggesting a unique function for SMARCAL1. These studies identified the first source of endogenous replication stress that SMARCAL1 resolves and provide insight into the mechanism of SMARCAL1 function in maintaining genome stability.
KEYWORDS: c-circle, DNA replication, DNA damage response, fork remodeling, replication stress, SMARCAL1, telomere
Introduction
With every round of DNA replication, the replication machinery is challenged by many endogenous and exogenous sources of stress. These stresses, if left unaddressed, can lead to detrimental effects including cell death or genetic instabilities that result in human disease.
One source of endogenous replication stress is difficult-to-replicate DNA sequences such as telomeres. These sequences are found at the ends of linear chromosomes and are composed of kilobases of a hexameric sequence repeat. Telomeres can challenge the rapid progression of the replication machinery due to their propensity to form complex DNA structures like G-quadruplexes.1 Specialized DNA and protein structures that protect the telomere, such as the t-loops, may also impair replisome movement. Furthermore, telomeres are origin-poor regions making replication fork stalling events especially problematic.2 To overcome these obstacles, specialized replication stress response proteins work to remove these sources of stress and promote the completion of DNA replication.
The SNF2 family of DNA translocases includes replication stress response proteins that work to stabilize and repair stalled replication forks. Within this family of enzymes, there are 3 closely related members: SMARCAL1 (SWI/SNF-related, matrix associated, actin-dependent, regulator of chromatin subfamily A-like 1), ZRANB3 (zinc finger, RAN-binding domain containing 3), and HLTF (helicase-like transcription factor). These translocases bind structured DNA and hydrolyze ATP.3-12 In vitro, all 3 enzymes can catalyze fork regression, which involves the reannealing of cDNA strands to convert a 3-way junction that mimics a replication fork structure into a 4-way junction.4,5,7-10,13 ZRANB3 and SMARCAL1 can also dissolve displacement loop structures and catalyze the restoration of a regressed, 4-way junction back to a normal replication fork structure.4,5,14
Despite the similarities in their biochemical activities, it is clear these proteins do not have redundant functions in cells. Depletion of each individual enzyme results in different cellular phenotypes.3,5,7,8,12,15-20 SMARCAL1 deficiency also causes the human disease Schimke immunoosseus dyslasia (SIOD)21 whereas the other proteins have not been directly implicated in a similar disease. Further, these proteins also differ in protein interactors. Of the 3, only SMARCAL1 has been shown to bind directly to the single-stranded DNA binding protein Replication Protein A (RPA).3,6,18,19 This interaction recruits SMARCAL1 to replication forks, regulates its enzymatic activity, and is necessary for at least some of its function in cells.3,6,14,18,20,22 ZRANB3 is also recruited to damaged forks,4 but via an interaction with ubiquitylated Proliferating Cell Nuclear Antigen (PCNA). Currently, the exact mechanism of HLTF recruitment is still unknown. Additionally, each of these proteins contains a distinctive substrate recognition domain that likely dictates their different substrate preferences.4,23,24 Thus, one hypothesis for the differences between these enzymes is that each may be specialized to resolve different types of replication stress. While experimental challenges with different DNA damaging or replication stress-inducing agents can provide some insights, identifying the endogenous sources of replication stress that these enzymes function to resolve is essential.
Recently, we reported that SMARCAL1 is needed to replicate telomeres and SMARCAL1-deficient cells exhibit telomere instability.25 Furthermore, this telomere replication function of SMARCAL1 is not shared by ZRANB3 or HLTF, providing the first evidence for the diverging functions of these SNF2 family enzymes in the resolution of endogenous sources of replication stress.
Identification of SMARCAL1 as a telomere maintenance protein
SMARCAL1 is required for replication fidelity. SMARCAL1-deficient cells accumulate damaged replication forks even without the addition of a genotoxic agent.3 Previous studies suggested that SMARCAL1 can form a complex with the WRN helicase.26 Since WRN is known to function to promote telomere replication, we hypothesized that these difficult-to-replicate sequences may also require SMARCAL1. Indeed, a subset of DNA damage foci that accumulate in SMARCAL1-deficient HeLa or mouse embryonic fibroblasts (MEFs) co-localize with telomeres. Only a fraction of telomeres in SMARCAL1-deficient cells display signs of damage at any one time, and these may represent the telomeres that are actively replicating.
Another marker of telomere instability, extrachromosomal, partially single-stranded telomere DNA circles (C-circles), also accompanies the telomere damage phenotype in SMARCAL1-deficient cells. Telomere replication is required for the C-circle accumulation in SMARCAL1-deficient cells indicating they form due to a replication problem that must be resolved by SMARCAL1. Importantly, we did not observe an accumulation of C-circles in HLTF- or ZRANB3-deficient cells.25 Nor did we observe C-circle accumulation in cells depleted of the RecQ helicases WRN or BLM.
The DNA damage that is observed in SMARCAL1-deficient cells is not limited to telomeres indicating SMARCAL1 is also required for replication of non-telomeric sequences.3,6,18,20 In bulk chromatin replication, the interaction with RPA helps recruit and regulate SMARCAL1.3,14 However, its function at telomeres does not require a direct interaction with RPA since expression of a SMARCAL1 mutant that cannot bind RPA rescues the telomere loss-of-function phenotype. It is possible that exogenous expression of SMARCAL1 increases its levels such that RPA no longer is required for SMARCAL1 recruitment to the forks in telomere regions. However, these results also are consistent with the idea that SMARCAL1 could recognize stalled forks in telomere sequences without RPA. As yet we have no evidence that SMARCAL1 binds a telomere binding protein as an alternative mode of recruitment. Perhaps SMARCAL1 simply utilizes its DNA binding capabilities to localize to replication forks in telomeres without requiring an additional recruitment factor.
Interestingly, we only observed the telomere defects associated with SMARCAL1 deficiency in cells with long telomeres such as MEFs and a long-telomere HeLa cell clone. A possible explanation is that longer telomere sequences present a greater opportunity for fork stalling. In other words, larger numbers of telomere repeats could provide a larger obstacle to the replisome and result in damage more often than shorter telomeres. This length dependence also has implications for the type of problem that is being resolved by SMARCAL1. For example, it may indicate that the t-loop that forms at the end of the chromosome is not the obstacle that SMARCAL1 resolves or that it is more of a problem in cells with long telomeres. The other likely obstacle that SMARCAL1 could resolve is DNA secondary structures like G-quadruplexes that form especially on the lagging strand in telomeres sequences. A lagging-strand problem could explain why an interaction with RPA is not needed since RPA is thought to activate SMARCAL1 only when bound to the leading strand template.14,22 However, we did not observe an increased sensitivity of SMARCAL1-deficient cells to G-quadruplex stabilizing agents like TMPyP4, so further studies will be needed to understand the exact nature of the telomere replication challenge that SMARCAL1 resolves.
SMARCAL1 and ALT
C-circles are closely associated with cells undergoing the recombination-based method of telomere maintenance known as alternative lengthening of telomeres (ALT).27 Measuring C-circle levels is one of the primary hallmarks and tests of ALT pathway utilization in cancer cells.28 The exact mechanism by which C-circles are generated in ALT cells is unknown. Furthermore, it is also unclear whether they are simply a byproduct of ALT or if they are important to act as templates for telomere elongation during ALT. The only other gene deficiency known to cause C-circle formation in telomerase-positive cells is the histone chaperone anti-silencing factor 1, ASF1.29 The increase in C-circles following ASF1 silencing is accompanied by other markers of ALT. ASF1-deficient cells display telomere length heterogeneity, elevated rates of telomere recombination, and increased frequencies of telomeres co-localized with promyelocytic leukemia (PML) in ALT-associated PML bodies (APBs).29 However, we were unable to detect any of these phenotypes in SMARCAL1-deficient cells.25 This is the first case, to our knowledge, of persistent C-circles without the presence of other ALT phenotypes. Thus, C-circles may not always indicate cells are using the ALT mechanism of telomere elongation.
Since our paper was published, another group also identified SMARCAL1 as a telomere maintenance protein.30 In this manuscript, SMARCAL1 was determined to be necessary for replication through telomeres exclusively in ALT cells. However, no mechanism was offered to explain the requirement for SMARCAL1 only in ALT-positive cells. We depleted SMARCAL1 in ALT-positive U2OS cells and saw no significant change in APBs, C-circles, or telomere damage—all phenotypic markers of ALT. We have shown that SMARCAL1 depletion in 2 telomerase-positive cells lines, HeLa cells and MEFs, results in an increase in telomere damage.25 Taken together, our data indicate SMARCAL1 function during telomere replication is a more universal requirement during telomere replication rather than an ALT-specific telomere repair pathway.
Conclusion
Our studies identified the first source of endogenous replication stress that a SNF2 family replication fork remodeling enzyme is needed to resolve. This SMARCAL1 function helps to prevent or resolve telomere damage during DNA replication (Fig. 1). Importantly, other enzymes in the same family including ZRANB3 and HLTF do not share the telomere stability function of SMARCAL1.
Figure 1.
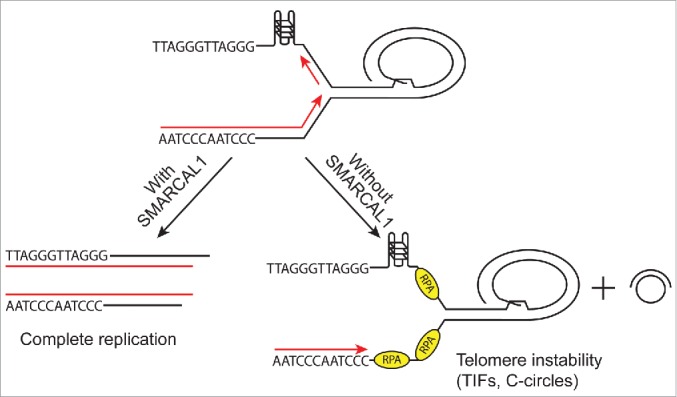
Model for SMARCAL1 function in maintaining telomere stability. SMARCAL1 ensures complete replication of telomere sequences, which are prone to form structures that can stall the replication fork. Telomere dysfunction-induced foci (TIFs) and extrachromosomal telomere circles (c-circles) accumulate in cells lacking SMARCAL1.
The exact mechanism of SMARCAL1 action at telomeres remains unknown. SMARCAL1 can catalyze fork reversal and fork restoration, so these are possible candidates for its function at telomere repeats.4,6,14 Elucidating the mechanism of C-circle formation would help in defining what SMARCAL1 does at telomeres as well as provide insights into the ALT mechanism. Other enzymes, such as the helicase RTEL1, also function to limit telomere instability and can perform similar in vitro activities as SMARCAL.31 Further studies to understand the cooperation between these telomere-associated enzymes in preventing telomere defects is needed. In addition, it is tempting to speculate that at least some of the clinical symptoms of SIOD such as bone growth defects and immunodeficiency could be linked to the need for SMARCAL1 to complete telomere replication. Animal models may help to answer this question. Finally, defining the other endogenous replication stress sources that SMARCAL1 and other related enzymes resolve is essential to fully understand how SMARCAL1 maintains genome integrity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Gilson E, Geli V. How telomeres are replicated. Nat Rev Mol Cell Biol 2007; 8:825-38; PMID:17885666; http://dx.doi.org/ 10.1038/nrm2259 [DOI] [PubMed] [Google Scholar]
- [2].Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 2009; 138:90-103; PMID:19596237; http://dx.doi.org/ 10.1016/j.cell.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bansbach CE, Betous R, Lovejoy CA, Glick GG, Cortez D. The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev 2009; 23:2405-14; PMID:19793861; http://dx.doi.org/ 10.1101/gad.1839909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Betous R, Mason AC, Rambo RP, Bansbach CE, Badu-Nkansah A, Sirbu BM, Eichman BF, Cortez D. SMARCAL1 catalyzes fork regression and Holliday junction migration to maintain genome stability during DNA replication. Genes Dev 2012; 26:151-62; PMID:22279047; http://dx.doi.org/ 10.1101/gad.178459.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ciccia A, Nimonkar AV, Hu Y, Hajdu I, Achar YJ, Izhar L, Petit SA, Adamson B, Yoon JC, Kowalczykowski SC, et al.. Polyubiquitinated PCNA recruits the ZRANB3 translocase to maintain genomic integrity after replication stress. Mol Cell 2012; 47:396-409; PMID:22704558; http://dx.doi.org/ 10.1016/j.molcel.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yusufzai T, Kong X, Yokomori K, Kadonaga JT. The annealing helicase HARP is recruited to DNA repair sites via an interaction with RPA. Genes Dev 2009; 23:2400-4; PMID:19793863; http://dx.doi.org/ 10.1101/gad.1831509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Achar YJ, Balogh D, Haracska L. Coordinated protein and DNA remodeling by human HLTF on stalled replication fork. Proc Natl Acad Sci U S A 2011; 108:14073-8; PMID:21795603; http://dx.doi.org/ 10.1073/pnas.1101951108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Blastyak A, Hajdu I, Unk I, Haracska L. Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA. Mol Cell Biol 2010; 30:684-93; PMID:19948885; http://dx.doi.org/ 10.1128/MCB.00863-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burkovics P, Sebesta M, Balogh D, Haracska L, Krejci L. Strand invasion by HLTF as a mechanism for template switch in fork rescue. Nucleic Acids Res 2014; 42:1711-20; PMID:24198246; http://dx.doi.org/ 10.1093/nar/gkt1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hishiki A, Hara K, Ikegaya Y, Yokoyama H, Shimizu T, Sato M, Hashimoto H. Structure of a Novel DNA-binding Domain of Helicase-like Transcription Factor (HLTF) and Its Functional Implication in DNA Damage Tolerance. J Biol Chem 2015; 290:13215-23; PMID:25858588; http://dx.doi.org/ 10.1074/jbc.M115.643643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].MacKay C, Toth R, Rouse J. Biochemical characterisation of the SWI/SNF family member HLTF. Biochem Biophys Res Commun 2009; 390:187-91; PMID:19723507; http://dx.doi.org/ 10.1016/j.bbrc.2009.08.151 [DOI] [PubMed] [Google Scholar]
- [12].Yuan J, Ghosal G, Chen J. The HARP-like domain-containing protein AH2/ZRANB3 binds to PCNA and participates in cellular response to replication stress. Mol Cell 2012; 47:410-21; PMID:22705370; http://dx.doi.org/ 10.1016/j.molcel.2012.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yusufzai T, Kadonaga JT. HARP Is an ATP-Driven Annealing Helicase. Science 2008; 322:748-50; PMID:18974355; http://dx.doi.org/ 10.1126/science.1161233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Betous R, Couch FB, Mason AC, Eichman BF, Manosas M, Cortez D. Substrate-selective repair and restart of replication forks by DNA translocases. Cell Rep 2013; 3:1958-69; PMID:23746452; http://dx.doi.org/ 10.1016/j.celrep.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lin JR, Zeman MK, Chen JY, Yee MC, Cimprich KA. SHPRH and HLTF act in a damage-specific manner to coordinate different forms of postreplication repair and prevent mutagenesis. Mol Cell 2011; 42:237-49; PMID:21396873; http://dx.doi.org/ 10.1016/j.molcel.2011.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Weston R, Peeters H, Ahel D. ZRANB3 is a structure-specific ATP-dependent endonuclease involved in replication stress response. Genes Dev 2012; 26:1558-72; PMID:22759634; http://dx.doi.org/ 10.1101/gad.193516.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yusufzai T, Kadonaga JT. Annealing helicase 2 (AH2), a DNA-rewinding motor with an HNH motif. Proc Natl Acad Sci U S A 2010; 107:20970-3; PMID:21078962; http://dx.doi.org/ 10.1073/pnas.1011196107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ciccia A, Bredemeyer AL, Sowa ME, Terret ME, Jallepalli PV, Harper JW, Elledge SJ. The SIOD disorder protein SMARCAL1 is an RPA-interacting protein involved in replication fork restart. Genes Dev 2009; 23:2415-25; PMID:19793862; http://dx.doi.org/ 10.1101/gad.1832309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Postow L, Woo EM, Chait BT, Funabiki H. Identification of SMARCAL1 as a component of the DNA damage response. J Biol Chem 2009; 284:35951-61; PMID:19841479; http://dx.doi.org/ 10.1074/jbc.M109.048330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yuan J, Ghosal G, Chen J. The annealing helicase HARP protects stalled replication forks. Genes Dev 2009; 23:2394-9; PMID:19793864; http://dx.doi.org/ 10.1101/gad.1836409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boerkoel CF, Takashima H, John J, Yan J, Stankiewicz P, Rosenbarker L, André JL, Bogdanovic R, Burguet A, Cockfield S, et al.. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat Genet 2002; 30:215-20; PMID:11799392; http://dx.doi.org/ 10.1038/ng821 [DOI] [PubMed] [Google Scholar]
- [22].Bhat KP, Betous R, Cortez D. High-affinity DNA-binding domains of replication protein A (RPA) direct SMARCAL1-dependent replication fork remodeling. J Biol Chem 2015; 290:4110-7; PMID:25552480; http://dx.doi.org/ 10.1074/jbc.M114.627083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Badu-Nkansah A, Mason AC, Eichman BF, Cortez D. Identification of a Substrate Recognition Domain in the Replication Stress Response Protein Zinc Finger Ran-binding Domain Containing Protein 3 (ZRANB3). J Biol Chem 2016; 291(15):8251-7; PMID:26884333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kile AC, Chavez DA, Bacal J, Eldirany S, Korzhnev DM, Bezsonova I, Eichman BF, Cimprich KA. HLTF's Ancient HIRAN Domain Binds 3' DNA Ends to Drive Replication Fork Reversal. Mol Cell 2015; 58:1090-100; PMID:26051180; http://dx.doi.org/ 10.1016/j.molcel.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Poole LA, Zhao R, Glick GG, Lovejoy CA, Eischen CM, Cortez D. SMARCAL1 maintains telomere integrity during DNA replication. Proc Natl Acad Sci U S A 2015; 112(48):14864-9; PMID:26578802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Betous R, Glick GG, Zhao R, Cortez D. Identification and characterization of SMARCAL1 protein complexes. PLoS One 2013; 8:e63149; PMID:23671665; http://dx.doi.org/ 10.1371/journal.pone.0063149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Henson JD, Cao Y, Huschtscha LI, Chang AC, Au AY, Pickett HA, Reddel RR. DNA C-circles are specific and quantifiable markers of alternative-lengthening-of-telomeres activity. Nat Biotechnol 2009; 27:1181-5; PMID:19935656; http://dx.doi.org/ 10.1038/nbt.1587 [DOI] [PubMed] [Google Scholar]
- [28].Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 2010; 11:319-30; PMID:20351727; http://dx.doi.org/ 10.1038/nrg2763 [DOI] [PubMed] [Google Scholar]
- [29].O'Sullivan RJ, Arnoult N, Lackner DH, Oganesian L, Haggblom C, Corpet A, Almouzni G, Karlseder J. Rapid induction of alternative lengthening of telomeres by depletion of the histone chaperone ASF1. Nat Struct Mol Biol 2014; 21:167-74; PMID:24413054; http://dx.doi.org/ 10.1038/nsmb.2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cox KE, Marechal A, Flynn RL. SMARCAL1 Resolves Replication Stress at ALT Telomeres. Cell Rep 2016; 14(5):1032-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vannier JB, Pavicic-Kaltenbrunner V, Petalcorin MI, Ding H, Boulton SJ. RTEL1 dismantles T loops and counteracts telomeric G4-DNA to maintain telomere integrity. Cell 2012; 149:795-806; PMID:22579284; http://dx.doi.org/ 10.1016/j.cell.2012.03.030 [DOI] [PubMed] [Google Scholar]


