Figure 2. Set up used to deliver spatio-temporally precise light stimulation of optogenetic ion-translocators expressed in Xenopus embryo ITLSs.
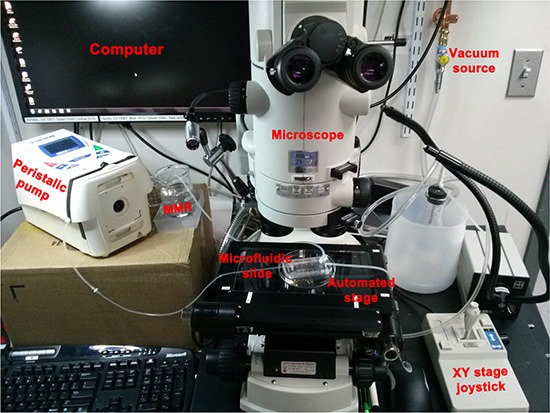
We customized a Nikon AZ100 dissection scope for in vivo optogenetics [87] by replacing the epifluorescence illumination source and light guide with a Spectra4 LED illuminator connected to the scope via fiber optic cable (the black light guide that passes in front of the vacuum source). The light is passed through a pinhole to set spot size diameter (located behind the oculars, not visible in this image), then enters the scope and goes through an 80/20 splitter that allows the user to view the specimen on the monitor even while the LED is on. Finally, the light passes through the 5 × objective lens which further reduces spot diameter and aims the spot at the sample. An automated Ludl MAC6000 XY stage (that can also be manipulated manually by a joystick) allows multiple embryos to be exposed repeatedly to the activating wavelength of light. Up to thirty embryos are loaded into a slide-mounted PDMS “chip” designed to use microfluidics to hold embryos in place [100]; the chip is held to the slide by a vacuum and 0.1 × MMR is circulated by a peristaltic pump. The optogenetics components and the microscope are all controlled by NIS Elements.
