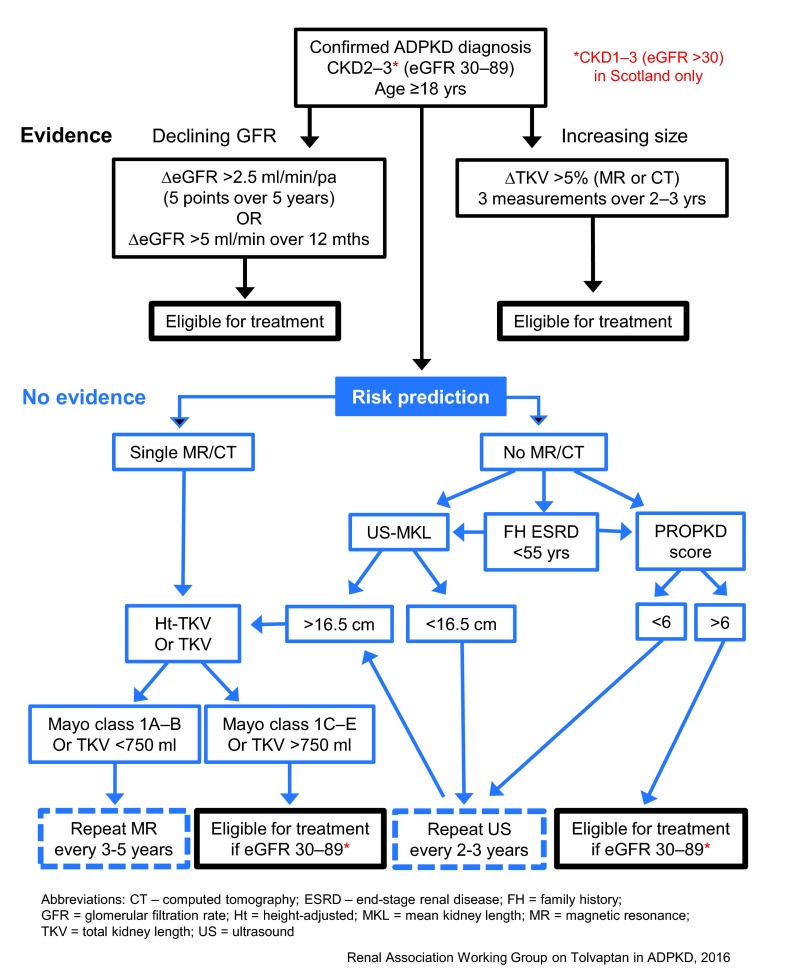
Figure 3. Recommended decision pathway for UK patients considered for treatment with tolvaptan according to Renal Association guidance based on the NICE decision.
NICE has recommended that autosomal dominant polycystic kidney disease patients in England with chronic kidney disease stages 2 or 3 and evidence of rapidly progressive disease are eligible for treatment. This decision differs from that made by the Scottish Medicines Consortium and the European Medicines Agency in excluding patients with chronic kidney disease stage 1. Evidence of rapid disease progression has been defined as a significant decline in estimated glomerular filtration rate or increase in total kidney volume or both. In the absence of such evidence, risk prediction algorithms based on total kidney volume (Mayo Classification) or genotype (PROPKD) are the best predictors of prognosis. NICE, National Institute for Health and Clinical Excellence.