Abstract
STUDY QUESTION
Is preconception urinary paracetamol (acetaminophen) associated with time-to-pregnancy (TTP)?
SUMMARY ANSWER
Higher urinary paracetamol concentrations among male partners were associated with a longer TTP.
WHAT IS KNOWN ALREADY
Paracetamol is a commonly used analgesic among women and men of all ages. As metabolites of select chemicals used in the manufacturing of polyurethane foam, dyes and various industrial products, as well as a common medicinal product, paracetamol and its primary metabolite p-aminophenol, are ubiquitous in the environment. Studies investigating the relationship between adult urinary concentrations of paracetamol and TTP are lacking.
STUDY DESIGN, SIZE, DURATION
This prospective cohort included 501 couples discontinuing contraception for the purposes of attempting conception during the years 2005–2009 and residing in Michigan or Texas, USA.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Total urinary paracetamol, its metabolite para-aminophenol (p-aminophenol), and a summary measure of both urinary biomarkers were quantified by ultra-performance liquid chromatography coupled with an electrospray triple quadrupole mass spectrometry (UPLC-ESI-MS/MS). Female partners used the Clearblue® digital home test to confirm pregnancy. Cox's proportional odds models for discrete survival time were used to estimate fecundability odds ratios (FORs) and 95% confidence intervals (CIs), adjusting for age, body mass index (BMI), urinary creatinine, preconception smoking status, race/ethnicity and household income. Models were further adjusted for hypothyroidism and hypertension as an attempt to account for possible indications of paracetamol medication use. FOR estimates <1.0 denote a longer TTP (diminished fecundity). Models were performed to examine urinary concentrations of paracetamol as a continuous and variable or categorized into quartiles. In light of TTP being a couple-dependent outcome, models were first performed for females and males, modeled separately, and then modeled for couples with each partner's concentrations being adjusted for the other.
MAIN RESULTS AND THE ROLE OF CHANCE
Among the 501 enrolled couples, 347 (69%) had an human chorionic gonadotrophin confirmed pregnancy. Urinary concentrations of paracetamol were lowest among females and males who achieved pregnancy and p-aminophenol concentrations were lowest among those not achieving pregnancy. Urinary paracetamol concentrations were higher among female than male partners (Median = 26.6 and 13.2 ng/ml, respectively; P < 0.0001). After adjustment for age, BMI, urinary creatinine, preconception smoking status, race/ethnicity and household income, the highest quartile of male urinary paracetamol was associated with a longer TTP [FOR = 0.67; 95% CI = (0.47, 0.95)]. This association remained after adjustment for chronic health conditions, hypothyroidism and hypertension and female partner's urinary paracetamol concentration [FOR = 0.65; 95% CI = (0.45, 0.94)]. No associations were observed between female or male partners' urinary concentrations of paracetamol or of its metabolite p-aminophenol when urinary concentrations were modeled continuously.
LIMITATIONS, REASONS FOR CAUTION
Only a single spot urine was available for analysis despite the short-lived nature of paracetamol. Additionally, participants were not asked to provide information on indication of use for paracetamol medications; any underlying conditions for the paracetamol use would have been potential confounders.
WIDER IMPLICATIONS OF THE FINDINGS
If corroborated with more robust studies, findings from our exploratory analysis may have both clinical and public health relevance among reproductive aged individuals, including those trying for pregnancy, given the prevalent use of paracetamol/acetaminophen medications and the ubiquitous nature of paracetamol in the environment.
STUDY FUNDING/COMPETING INTEREST(S)
This research was supported by the National Institutes of Health, Intramural Research Program, and Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts N01-HD-3-3355; N01-HD-3-3356; NOH-HD-3-3358; HHSN27500001/HHSN27500001). None of the authors have any conflicts to declare.
Keywords: acetaminophen, biomarkers, fecundity, paracetamol, p-aminophenol, TTP
Introduction
Public health concerns have increased regarding the potential role of environmental chemicals, such as persistent organic pollutants, phthalates and heavy metals, as important contributors to male, female and couple fecundity (Eskenazi et al., 2010; Harley et al., 2010; Bloom et al., 2011; Buck Louis et al., 2012; Buck Louis et al., 2013; Specht et al., 2015; Velez et al., 2015a,b). Like many of these environmental chemicals, paracetamol is ubiquitous in contemporary environments with routes of exposures stemming from health status (e.g. medicinal use for chronic musculoskeletal pain) and other environmental sources (e.g. drinking water).
As commonly used analgesic and antipyretic, paracetamol (also known as acetaminophen in the USA and Canada) is found in a large number of over-the-counter (OTC) and prescription drugs (Lank, 2014). Approximately 30–70% of pregnant women report having taken acetaminophen/paracetamol at least once during pregnancy when queried in (inter)national epidemiologic studies (Werler et al., 2005; Rebordosa et al., 2008), underscoring the ubiquitous exposure for reproductive aged populations. Furthermore, low levels of paracetamol have been detected in drinking water (Wu et al., 2012). Interestingly, aniline, a chemical used in the synthesis and manufacturing of many industrial products (e.g. dyes, polyurethane foam, rubber processing chemicals) (ATSDR, 2011), metabolizes into p-aminophenol and paracetamol. As a reflection of their environmentally abundance, urinary concentrations of paracetamol and an isomer of p-aminophenol have been readily detected at levels as high as 2 263 and 39 161 μg/l, respectively, in a general European population without occupational or medicinal exposure (Dierkes et al., 2014).
Paracetamol readily crosses the placental barrier (Gaber, 2006). Due to associations with increased percentages of abnormal sperm, reduced anogential distance and decreased testosterone production in rodent studies (Kristensen et al., 2011, 2012; Thiele et al., 2013), paracetamol is suspected to have endocrine disrupting properties. In human studies, a Danish prospective birth cohort study reported that compared with mothers who reported no medication use during pregnancy, women who used paracetamol for more than 2 weeks during the first and second trimesters had an increased risk of delivering boys with congenital cryptorchidism (Kristensen et al., 2011). Other European studies of paracetamol use assessed during pregnancy have reported similar findings, with significant associations between maternal paracetamol use and male reproductive disorders (Jensen et al., 2010; Philippat et al., 2011; Snijder et al., 2012).
To our knowledge, despite the growing body of literature surrounding exposure and reproductive considerations, the relationship between paracetamol and sensitive measures of human fecundity, such as time-to-pregnancy (TTP) or the number of menstrual cycles or calendar months required to achieve pregnancy, has not been previously explored. We sought to prospectively assess male and female partners' urinary concentrations of paracetamol and TTP in the present analysis.
Materials and Methods
Study population and participants
The Longitudinal Investigation of Fertility and the Environment (LIFE) Study comprises 501 reproductive aged couples who were recruited upon discontinuing contraception for ≤2 months prior to enrollment, for purposes of becoming pregnant. Couples were recruited from 16 counties in Michigan and Texas between 2005 and 2009, as previously described (Buck Louis et al., 2011). Inclusion criteria were minimal: females aged 18–40 and males aged ≥18 years; in a committed relationship; no physician diagnosis of infertility/sterility; females' menstrual cycles ranging between 21 and 42 days without any injectable hormonal contraceptives in the past year; and an ability to communicate in English or Spanish.
Ethical approval
Institutional review board approvals were obtained from all collaborating institutions; couples gave written informed consent prior to study participation and any data collection.
Urinary analysis of paracetamol and its metabolite
After the baseline interview during the enrollment home visit, couples provided a non-fasting urine sample. Chemical analysis of total urinary paracetamol and p-aminophenol was performed at the Wadsworth Center, New York State Department of Health, Albany, NY. Specifically, 300 µl of 1 M ammonium acetate containing 30 U of β-glucuronidase (pH = 5.5) was added to 500 µl of urine sample, followed by incubation at 37°C for 12 h. Target analytes were extracted thrice with ethyl acetate and were quantified by ultra-high performance liquid chromatography (Acquity I Class; Waters, Milford, MA, USA) coupled with an electrospray triple quadrupole mass spectrometry (API 5500; AB SCIEX, Framingham, MA, USA) (UPLC-ESI-MS/MS). Separation of target analytes was carried out by a Kinetex C18 (1.3 µ, 100A, 50 × 2.1 mm) column (Phenomenex; Torrance, CA, USA) with a SecurityGuard guard column (Phenomenex) and positive ionization, multiple reaction monitoring mode of detection. Quality assurance and quality control parameters included procedural blanks, matrix spikes and duplicate analysis of samples. Labeled internal standards (d7-p-aminophenol and 13C2-15N-paracetamol) were spiked into all samples and quantification was by isotope dilution. The method limits of quantification (LOQ) for p-aminophenol and paracetamol were 0.25 and 0.5 ng/ml, respectively. Creatinine was quantified using a Roche/Hitachi Model 912 clinical analyzer (Dallas, TX, USA) and the Creatinine Plus Assay. In addition, non-fasting blood samples were collected from each partner for the measurement of serum cotinine using liquid chromatography-isotope dilution tandem mass spectrometry and the results are reported in ng/ml (Bernert et al., 1997).
Lifestyle and TTP data collection
Upon enrollment, in-person interviews were conducted with each partner of the couple to ascertain lifestyle and reproductive history followed by standard anthropometric assessments to measure body mass index (BMI) (Lohman et al., 1988). Daily journals were completed by both partners to capture various lifestyle behaviors relevant to fecundity and sexual intercourse; women's journals also recorded menstruation and pregnancy test results. Women were instructed in the use of the Clearblue® Easy home fertility monitor to track daily concentrations of estrone-3-glucuronide and luteinizing hormones to help time intercourse relative to ovulation. The fertility monitor is accurate in detecting the luteinizing hormone surge (99%) and in predicting peak fertility (91%) compared with the ultrasonography gold standard (Behre et al., 2000). Additionally, female partners were instructed in the use of the Clearblue® digital pregnancy test, which is capable of detecting 25 mIU/ml human chorionic gonadotrophin (hCG) when used on the day of expected menstruation. Urine for each woman was tested at baseline to ensure the absence of pregnancy. The Clearblue® Digital home pregnancy test is capable of detecting pregnancy with 99% accuracy when used from the day of expected menstrual cycle (Tomlinson et al., 2008). Couples achieving pregnancy in the first few weeks post-enrollment were categorized as having a TTP = 0 and those achieving pregnancy during the first fully observed cycle were categorized as having a TTP = 1. Couples were followed until pregnancy or a year of trying, at which point TTP was censored.
Statistical analysis
Univariate analyses were performed to assess the distributions of paracetamol, its metabolite p-aminophenol and relevant covariates. Female and male lifestyle characteristics were compared by observed pregnancy status using independent t-tests and χ2 tests for continuous and categorical covariates, respectively. Correlations between female and male partner's paracetamol and p-aminophenol urinary concentrations were examined with the use of Spearman rank analyses. Median and accompanying interquartile ranges (IQRs) of baseline urinary chemical concentrations (ng/ml) were calculated; medians were compared between females and males using Wilcoxon–Mann–Whitney tests. To avoid biasing regression estimates, instrument-derived values (i.e. baseline noise) for both chemicals were used in all models with urinary creatinine included as a covariate; urinary concentrations below the LOQ were not substituted in any manner (Richardson and Ciampi, 2003; Lubin et al., 2004; Schisterman et al., 2006). In the event of missing chemical data as the result of insufficient female (6%) and male (12%) urine for chemical quantification, data were imputed with Box–Cox transformation, using Markov Chain Monte Carlo methods (Schafer, 1997). Urinary creatinine and chemical concentrations were natural-log transformed (ln) to normalize distributions.
Cox proportional odds models for discrete survival time were used to estimate fecundability odds ratios (FORs) and 95% CIs, which allows for a cycle-varying intercept (Cox, 1972). Models accounted for left truncation reflecting the time couples had discontinued contraception, and also right censoring as couples exited the study or reached 1 year of trying. FORs estimate the odds of becoming pregnant each cycle, conditional on not being pregnant in the previous cycle. FOR estimates <1.0 reflect a longer TTP suggestive of diminished fecundity, while FORs >1 reflect a shorter TTP or enhanced fecundity. Urinary concentrations of paracetamol, p-aminophenol, and a summary measure of both urinary biomarkers were first modeled individually for each partner and adjusted based on a priori selection of confounders: non-time-varying age (years), BMI (25 ≤ BMI < 30, 30 ≤ BMI < 35 and BMI ≥ 35 kg/m2 compared with BMI < 25 kg/m2), urinary creatinine (ng/ml), preconception smoking status (serum cotinine above/below10 ng/ml indicative of active smoking or not (Hukkanen et al., 2005; Benowitz et al., 2009)), race/ethnicity (non-White/White) and household income (above/below $70 000). We refer to these models as partner-specific models of TTP. Partner-specific models were further adjusted for chronic health conditions, namely, hypothyroidism (Wilson and Walton, 1959; Golding, 1970; Cakir et al., 2003) and hypertension (Lawrence, 1975; Kerkhoff et al., 2012; Bae et al., 2015), in an attempt to control for differences in the use of paracetamol medication. In light of low correlation between both partners' biomarker levels (r= 0.11 for paracetamol and r= 0.10 for p-aminophenol), we re-ran models with the same covariates including the urinary concentrations of the other partner to assess TTP as a couple-dependent outcome. Couple-based models of TTP were adjusted to account for both partners' covariates and the difference between female and male age (years). Furthermore, models were run first with urinary concentrations left continuous. To assess potential nonlinear associations, models were performed with urinary paracetamol and p-aminophenol concentrations categorized into quartiles. Wald tests were performed to test for nonlinear trend across quartiles of chemical concentrations.
As a sensitivity analysis, TTP was modeled against non-imputed urinary concentrations of paracetamol and p-aminophenol to determine whether the obtained results were different from the primary analyses. Given the exploratory nature of this study and the bias introduced to point estimates of association when excluding participants with missing information (White and Carlin, 2010; Desai et al., 2011), we focused on the imputed data in the primary analysis to reduce the number of comparisons and preserve our type I error rate.
Results
Among the 501 enrolled couples, 347 (69%) had an observed hCG confirmed pregnancy within a year. The cohort comprised mostly non-Hispanic White couples with household incomes ≥$70 000 (Table I). Females and males achieving pregnancy tended to be younger in age, had lower BMIs, were more parous and had a lower prevalence of preconception smoking compared with those not achieving pregnancy during the study. Characteristics of females and males who withdrew from the study (n = 100, 20%) were similar with those not achieving pregnancy during the study (Table I).
Table I.
Cohort characteristics by observed pregnancy status, LIFE Study (2005–2009)a.
| Characteristic | Pregnant (n = 347) | Not pregnant (n = 54) | Withdrew (n = 100) |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Female age (years) | 29.8 ± 3.9 | 30.6 ± 4.3 | 30.3 ± 4.8 |
| Male age (years) | 31.6 ± 4.6 | 32.4 ± 5.3 | 32.1 ± 5.8 |
| Female BMI (kg/m2) | 27.0 ± 6.7 | 28.7 ± 9.1 | 29.4 ± 7.9 |
| Male BMI (kg/m2) | 29.7 ± 5.5 | 30.3 ± 5.5 | 29.9 ± 5.8 |
| Gravidity (# pregnancies) | 1.1 ± 1.2 | 0.8 ± 1.3 | 1.3 ± 1.5 |
| Parity (# live births)*** | 0.7 ± 0.8 | 0.2 ± 0.5 | 0.7 ± 1.0 |
| n (%) | n (%) | n (%) | |
| Female race/ethnicity | |||
| Non-Hispanic white | 285 (82) | 39 (72) | 66 (66) |
| Non-Hispanic black | 5 (1) | 4 (7) | 13 (13) |
| Hispanic | 29 (8) | 8 (15) | 13 (13) |
| Other | 28 (8) | 3 (6) | 7 (7) |
| Male race/ethnicity | |||
| Non-Hispanic white | 278 (80) | 42 (78) | 64 (64) |
| Non-Hispanic black | 7 (2) | 4 (7) | 12 (12) |
| Hispanic | 30 (9) | 6 (11) | 12 (12) |
| Other | 10 (3) | — | 3 (3) |
| Female smoking** | |||
| No | 308 (92) | 45 (79) | 78 (80) |
| Yes | 58 (12) | 12 (21) | 19 (20) |
| Male smoking** | |||
| No | 282 (83) | 43 (75) | 62 (65) |
| Yes | 58 (17) | 14 (25) | 34 (35) |
| Household income | |||
| <70 000 | 91 (27) | 24 (44) | 44 (44) |
| ⩾70 000 | 250 (73) | 30 (56) | 55 (55) |
*P < .001; **P < 0.0001.
aMissing data are not reflected in the table.
The comparisons of urinary paracetamol and p-aminophenol distributions for female and male partners by pregnancy status are displayed in Table II. Overall, paracetamol and p-aminophenol were readily detected in both male and female urine samples, with the percentage of urine samples with measurable concentrations ranging between 93 and 100%. Urinary concentrations of paracetamol were lowest among females and males who achieved pregnancy, and p-aminophenol concentrations were lowest among those who did not achieve pregnancy (Table II). When examining urinary concentrations of paracetamol by covariates of interest, geometric means differed by race/ethnicity and smoking status at baseline among female urine samples (Supplementary data, Table SI). Additionally, mean urinary p-aminophenol differed by BMI and household income levels. Overall, urinary paracetamol concentrations were higher for females than for males [Median = 26.6 and 13.2 ng/ml, respectively; P < 0.0001]. Similarly, the median urinary p-aminophenol was higher in females [Median = 957; IQR = (446, 2190) ng/ml], compared with male partners [Median = 726; IQR = (405, 1378) ng/ml; P = 0.000].
Table II.
Urinary paracetamol and p-aminophenol concentrations by pregnancy status, LIFE Study, 2005–2009.
| Biomarker (ng/ml) | % > LOQ | Pregnant | Not pregnant | Withdrew | P |
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |||
| Females, n | 334 | 50 | 86 | ||
| paracetamol (LOQ = 0.50 ng/ml) | 97 | 18.9 (6.49, 130) | 29.6 (5.75, 1301) | 20.2 (5.98, 253) | 0.75 |
| p-aminophenol (LOQ = 0.25 ng/ml) | 100 | 747 (305, 1282) | 649 (3145, 1793) | 939 (328, 1995) | 0.09 |
| Males, n | 313 | 48 | 78 | ||
| paracetamol (LOQ= 0.50 ng/ml) | 93 | 13.9 (5.25, 53.8) | 17.23 (6.54, 789) | 17.4 (6.19, 118) | 0.18 |
| p-aminophenol (LOQ = 0.25 ng/ml) | 100 | 979 (493, 1596) | 936 (508, 1476) | 978 (542, 1630) | 0.99 |
P-values were calculated using the nontransformed median in Kruskal–Wallis tests comparing urinary paracetamol and p-aminophenol concentrations across the three pregnancy status groups.
LOQ, limit of quantification; IQR, interquartile range.
Table III presents the associations between continuous and quartile models of urinary paracetamol biomarkers and TTP for partner-specific analyses. Among female partners, FORs were often greater than the null, although no association was observed between preconception urinary concentrations of paracetamol, p-aminophenol or the summary measure of urinary paracetamol biomarkers and TTP. Regarding male partners, a consistent association was observed in models of male urinary paracetamol and TTP (Table III). The highest quartile (>73.5 ng/ml) of male urinary paracetamol was associated with delayed TTP compared with the lowest quartile (<5.44 ng/ml) after adjustment for age, BMI, creatinine, smoking status, race/ethnicity and income [aFOR = 0.67; 95% CI = (0.47, 0.95); P-trend = 0.02] and further adjustment for hypertension and hypothyroidism [aFOR = 0.65; 95% CI = (0.45, 0.92); P-trend = 0.02].
Table III.
Associations between urinary paracetamol and p-aminophenol concentrations and TTP in partner-specific models (n = 501).
| Partner-specific models |
|||
|---|---|---|---|
| Unadjusted | Adjusteda | Adjustedb | |
| FOR (95% CI) | FOR (95% CI) | FOR (95% CI) | |
| Females | |||
| Paracetamolc | 1.00 (0.96, 1.04) | 1.01 (0.97, 1.06) | 1.01 (0.97, 1.05) |
| Quartile 1: <6.25 ng/ml | — | — | — |
| Quartile 2: 6.25–19.95 ng/ml | 1.22 (0.88, 1.70) | 1.18 (0.84, 1.65) | 1.22 (0.86, 1.72) |
| Quartile 3: 19.95–157 ng/ml | 1.16 (0.82, 1.63) | 1.26 (0.88, 1.80) | 1.38 (0.96, 2.00) |
| Quartile 4: >157 ng/ml | 1.02 (0.71, 1.46) | 1.11 (0.76, 1.61) | 1.34 (0.89, 2.01) |
| P-trend | 0.93 | 0.60 | 0.60 |
| p-aminophenolc | 0.94 (0.86, 1.04) | 0.95 (0.86, 1.05) | 0.98 (0.88, 1.10) |
| Quartile 1: <315 ng/ml | — | — | — |
| Quartile 2: 315–781 ng/ml | 1.19 (0.85, 1.66) | 1.26 (0.87, 1.81) | 1.29 (0.88, 1.89) |
| Quartile 3: 781–1403 ng/ml | 1.33 (0.95, 1.85) | 1.37 (0.96, 1.95) | 1.41 (0.98, 2.03) |
| Quartile 4: >1403 ng/ml | 0.75 (0.53, 1.05) | 0.91 (0.62, 1.35) | 0.93 (0.61, 1.40) |
| P-trend | 0.10 | 0.64 | 0.71 |
| Paracetamol + p-aminophenolc | 1.00 (0.96, 1.04) | 1.01 (0.97, 1.05) | 1.01 (0.97, 1.05) |
| Quartile 1: <410 ng/ml | — | — | — |
| Quartile 2: 410–1020 ng/ml | 0.98 (0.70, 1.37) | 1.02 (0.72, 1.45) | 1.03 (0.73, 1.47) |
| Quartile 3: 1020–2196 ng/ml | 1.11 (0.80, 1.56) | 1.28 (0.90, 1.82) | 1.28 (0.90, 1.82) |
| Quartile 4: >2196 ng/ml | 0.73 (0.51, 1.03) | 0.87 (0.60, 1.27) | 0.88 (0.60, 1.28) |
| P-trend | 0.07 | 0.46 | 0.50 |
| Males | |||
| Paracetamolc | 0.99 (0.96, 1.02) | 0.99 (0.96, 1.02) | 0.99 (0.96, 1.02) |
| Quartile 1: <5.44 ng/ml | — | — | — |
| Quartile 2: 5.44–15.52 ng/ml | 0.88 (0.62, 1.23) | 0.91 (0.64, 1.30) | 0.92 (0.63, 1.33) |
| Quartile 3: 15.52–73.47 ng/ml | 0.97 (0.69, 1.36) | 0.94 (0.65, 1.37) | 0.93 (0.63, 1.37) |
| Quartile 4: >73.47 ng/ml | 0.69 (0.49, 0.97) | 0.67 (0.47, 0.95) | 0.65 (0.45, 0.94) |
| P-trend | 0.03 | 0.02 | 0.02 |
| p-aminophenolc | 0.92 (0.85, 1.00) | 0.92 (0.85, 1.00) | 0.90 (0.81, 1.01) |
| Quartile 1: <500 ng/ml | — | — | — |
| Quartile 2: 500–978 ng/ml | 0.93 (0.66, 1.32) | 0.91 (0.63, 1.31) | 0.93 (0.63, 1.37) |
| Quartile 3: 978–1596 ng/ml | 1.00 (0.70, 1.41) | 1.03 (0.69, 1.54) | 1.04 (0.69, 1.58) |
| Quartile 4: >1596 ng/ml | 0.86 (0.60, 1.23) | 0.82 (0.53, 1.26) | 0.88 (0.55, 1.41) |
| P-trend | 0.41 | 0.36 | 0.43 |
| Paracetamol + p-aminophenolc | 0.99 (0.96, 1.02) | 0.99 (0.96, 1.02) | 0.99 (0.95, 1.02) |
| Quartile 1: <606 ng/ml | — | — | — |
| Quartile 2: 606–1114 ng/ml | 1.12 (0.79, 1.58) | 1.04 (0.72, 1.51) | 1.04 (0.71, 1.51) |
| Quartile 3: 1114–2029 ng/ml | 1.09 (0.76, 1.56) | 1.06 (0.71, 1.59) | 1.08 (0.72, 1.62) |
| Quartile 4: >2029 ng/ml | 0.76 (0.54, 1.07) | 0.69 (0.46, 1.02) | 0.70 (0.47, 1.04) |
| P-trend | 0.12 | 0.06 | 0.02 |
P-trend from Wald test for nonlinearity.
aModels were adjusted for age, BMI, creatinine, smoking status (cotinine dichotomized at a threshold of 10 ng/ml), race/ethnicity (dichotomized, non-White versus White) and income (dichotomized at $70 000).
bModels included covariates in model a and hypertension and hypothyroidism.
cModeled as continuous variable.
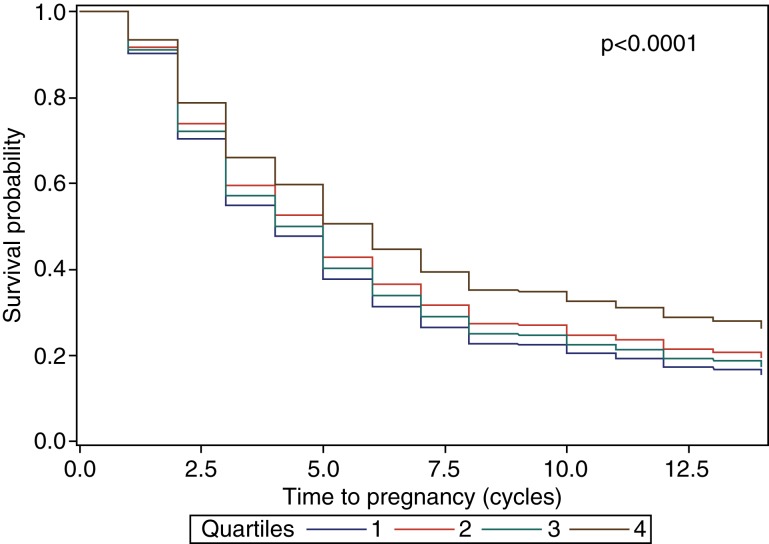
In Table IV, adjustment for both partner's urinary biomarkers and confounders resulted in similar findings to those from the partner-specific models (Table III). Again, no association was observed between female urinary paracetamol and TTP. Nonetheless, the fourth quartile of male partners' urinary paracetamol was associated with delayed TTP in the adjusted couple-based models [a FOR = 0.65; 95% CI = (0.47, 0.98); P-trend = 0.02]. No associations were observed when couples' urinary biomarkers were modeled continuously. Figure 1 demonstrates that the probability of remaining non-pregnant tended to be higher among couples whose male partners had urinary paracetamol concentrations in the fourth versus first quartile.
Table IV.
Associations between urinary paracetamol and p-aminophenol concentrations and TTP in couples (n = 501).
| Couple-based models |
|||
|---|---|---|---|
| Unadjusteda | Adjustedb | Adjustedc | |
| FOR (95% CI) | FOR (95% CI) | FOR (95% CI) | |
| Females | |||
| Paracetamold | 1.00 (0.96, 1.05) | 1.01 (0.97, 1.06) | 1.01 (0.97, 1.06) |
| Quartile 1: <6.25 ng/ml | — | — | — |
| Quartile 2: 6.25–19.95 ng/ml | 1.25 (0.89, 1.74) | 1.21 (0.86, 1.71) | 1.22 (0.86, 1.72) |
| Quartile 3: 19.95–157 ng/ml | 1.20 (0.86, 1.69) | 1.36 (0.95, 1.97) | 1.38 (0.96, 2.00) |
| Quartile 4: >157 ng/ml | 1.11 (0.75, 1.63) | 1.30 (0.87, 1.93) | 1.34 (0.89, 2.01) |
| P-trend | 0.6 | 0.19 | 0.16 |
| p-aminophenold | 0.95 (0.86, 1.05) | 1.00 (0.89, 1.13) | 1.00 (0.89, 1.13) |
| Quartile 1: <315 ng/ml | — | — | — |
| Quartile 2: 315–781 ng/ml | 1.19 (0.85, 1.66) | 1.31 (0.90, 1.91) | 1.29 (0.88, 1.89) |
| Quartile 3: 781–1403 ng/ml | 1.34 (0.96, 1.87) | 1.42 (0.99, 2.05) | 1.41 (0.98, 2.03) |
| Quartile 4: >1403 ng/ml | 0.76 (0.53, 1.08) | 0.96 (0.63, 1.45) | 0.93 (0.61, 1.40) |
| P-trend | 0.13 | 0.83 | 0.72 |
| Paracetamol + p-aminophenold | 1.00 (0.96, 1.04) | 1.01 (0.97, 1.05) | 1.01 (0.97, 1.05) |
| Quartile 1: <410 ng/ml | — | — | — |
| Quartile 2: 410–1020 ng/ml | 1.00 (0.72, 1.40) | 1.05 (0.73, 1.50) | 1.04 (0.71, 1.53) |
| Quartile 3: 1020–2196 ng/ml | 1.15 (0.83, 1.61) | 1.36 (0.94, 1.96) | 1.09 (0.71, 1.66) |
| Quartile 4: >2196 ng/ml | 0.75 (0.53, 1.08) | 0.92 (0.62, 1.35) | 0.69 (0.46, 1.03) |
| P-trend | 0.12 | 0.66 | 0.62 |
| Males | |||
| Paracetamold | 0.99 (0.96, 1.02) | 0.99 (0.96, 1.02) | 0.99 (0.96, 1.02) |
| Quartile 1: <5.44 ng/ml | — | — | — |
| Quartile 2: 5.44–15.52 ng/ml | 0.87 (0.62, 1.23) | 0.92 (0.64, 1.32) | 0.92 (0.63, 1.33) |
| Quartile 3: 15.52–73.47 ng/ml | 0.96 (0.68, 1.36) | 0.92 (0.63, 1.34) | 0.93 (0.63, 1.37) |
| Quartile 4: >73.47 ng/ml | 0.68 (0.47, 0.98) | 0.65 (0.45, 0.94) | 0.65 (0.45, 0.94) |
| P-trend | 0.04 | 0.02 | 0.02 |
| p-aminophenold | 0.92 (0.85, 1.00) | 0.90 (0.80, 1.01) | 0.90 (0.80, 1.01) |
| Quartile 1: <500 ng/ml | — | — | — |
| Quartile 2: 500–978 ng/ml | 0.95 (0.66, 1.35) | 0.92 (0.63, 1.35) | 0.93 (0.63, 1.37) |
| Quartile 3: 978–1596 ng/ml | 1.02 (0.72, 1.45) | 1.04 (0.69, 1.57) | 1.04 (0.69, 1.58) |
| Quartile 4: >1596 ng/ml | 0.90 (0.62, 1.31) | 0.86 (0.54, 1.37) | 0.88 (0.55, 1.41) |
| P-trend | 0.6 | 0.53 | 0.60 |
| Paracetamol + p-aminophenold | 0.99 (0.96, 1.02) | 0.99 (0.95, 1.02) | 0.99 (0.95, 1.02) |
| Quartile 1: <606 ng/ml | — | — | — |
| Quartile 2: 606–1114 ng/ml | 1.13 (0.80, 1.59) | 1.04 (0.71, 1.53) | 1.03 (0.70, 1.52) |
| Quartile 3: 1114–2029 ng/ml | 1.12 (0.78, 1.60) | 1.09 (0.71, 1.66) | 1.10 (0.71, 1.70) |
| Quartile 4: >2029 ng/ml | 0.78 (0.55, 1.10) | 0.69 (0.46, 1.03) | 0.69 (0.46, 1.04) |
| P-trend | 0.16 | 0.07 | 0.08 |
P-trend from Wald test for nonlinearity.
aModels included concentration for paracetamol or p-aminophenol and partner's concentration as well.
bModels were adjusted for female age, female and male age difference, female and male: BMI, creatinine, smoking status (cotinine dichotomized at a threshold of 10 ng/ml), race/ethnicity (dichotomized, non-White versus White) and income (dichotomized at $70 000).
cModels were adjusted for covariates listed in model B along with female and male hypertension and hypothyroid.
dModeled as continuous variable
Figure 1.
Probability of couples remaining not pregnant by male urinary paracetamol quartiles. Adjusted for age, BMI, creatinine, smoking status (cotinine dichotomized at a threshold of 10 ng/ml), race/ethnicity (dichotomized, non-White versus White), income (dichotomized at $70 000), hypertension and hypothyroidism. Quartile 1: <5.44 ng/ml; Quartile 2: 5.44–15.52 ng/ml; Quartile 3: 15.52–73.47 ng/ml; Quartile 4: > 73.47 ng/ml. Reported P-value for Likelihood Ratio and Wald comparisons between survival curves.
In sensitivity analyses restricted to females and males without missing urinary concentrations of paracetamol biomarkers (n= 435; 87%), the third quartile (19.95–157 ng/ml) of female partners' urinary paracetamol was associated with a shorter TTP (enhanced fecundity), in partner-specific and couple-based models controlling for demographic and chronic health confounders (Supplementary data, Table SII). When the analyses were restricted to males without missing chemical data, the observed association with the fourth quartile of urinary paracetamol remained although it was slightly attenuated (Supplementary data, Table SII).
Discussion
These are the first data known to have assessed preconception urinary concentrations of total paracetamol and its metabolite, p-aminophenol, in relation to TTP, reflective of couple fecundity. Paracetamol concentrations measured in urine for our study were readily detected in both women and men with detection frequencies well above 90%. We found male urinary concentrations of paracetamol in the highest quartile (>73.5 ng/ml) to be consistently associated with a longer TTP across all partner-specific and couple-based models. In contrast, female urinary biomarkers were not associated with TTP in all primary partner-specific or couple-based models.
Interpretation of our findings in relation to other populations is challenging due to the dearth of literature regarding the quantification of paracetamol in preconception urine and prospectively assessed TTP. As such, these findings may potentially be of clinical and public health relevance from two perspectives. First, there is a prevalent use of paracetamol globally as an OTC medication among men and women of all ages (Kaufman et al., 2002) including children 0 months to 12 years of age (Vernacchio et al., 2009). Second paracetamol and p-aminophenol measured in urine may also be the result of exposure to other ubiquitous chemicals in the environment. Specifically, p-aminophenol is a primary metabolite of aniline which is used in the manufacturing of various industrial products (Dierkes et al., 2014), and in other industries, e.g. meat production (Modick et al., 2014). The high detection frequency of paracetamol in our cohort among both men and women (93 and 97%, respectively) from two geographically different states may corroborate the variety of potential sources of paracetamol exposure.
In light of the high detection frequency of paracetamol in urine and the stark gender differences in urinary concentrations of paracetamol, with the highest distribution being among the females in the LIFE study, the lack of association between female urinary concentrations of paracetamol and TTP is worthy of further consideration. One possible explanation could be that chronic medical conditions resulting in paracetamol use may differ by gender and these conditions may also have the potential to directly affect human fecundity. Such information on chronic health conditions associated with chronic pain was not collected. As such, our model adjustment for hypothyroidism or hypertension did not completely address indication for pain medication but more so other medications (e.g. hormone therapy, antihypertensive drugs), as seen by the slightly attenuated observed male findings and the lack of change regarding our conclusions for female results. Another possibility to consider is that although females had higher measured concentrations of urinary paracetamol, perhaps in the context of human health, the source of paracetamol exposure may be of more importance than the magnitude of measured concentrations. Therefore, the observed associations may be confounded by differences in paracetamol exposure source; such information (e.g. self-report of occupational versus environmental exposures) was not collected and therefore was not included in regression models. Future research might consider source apportionment analyses in order to further understand the relationship between paracetamol and couple fecundity. While we were unable to perform such work, we did include an aggregate exposure measure (sum of both urinary paracetamol biomarkers) in relation to TTP, as an attempt to account for a multitude of potential exposure sources.
Despite the fact that we consistently observed an association among male, but not female, urinary paracetamol and TTP, there are several study design and analytic strengths to our analysis. First is the LIFE Study's prospective assessment of couples' TTP, which is comparable with the few other prospective cohort studies of TTP (Bonde et al., 1998; Gnoth et al., 2003; Mikkelsen et al., 2009; Steiner et al., 2011; Wise et al., 2015). In the LIFE Study, 69% of couples achieved hCG confirmed pregnancies, of which 90% occurred within six menstrual cycles. Secondly, we are the first to quantify urinary metabolite, p-aminophenol and total paracetamol in both partners at the time that they began trying for pregnancy. Thirdly, we do not believe that model estimates were heavily influenced by exposure misclassification given that our analyses relied on measured paracetamol in urine, rather than the low prevalence of the self-reported paracetamol use (2.6 and 1.2% for females and males in LIFE, respectively). Lastly, our use of Cox proportional odds models for discrete survival with a time-varying intercept minimizes information bias by utilizing all information provided by study participants, while under follow-up and prior to censoring, a single established protocol was used for quantifying all urine samples while being blinded to TTP.
Still, we must also acknowledge the potential limitations of the present analysis. Regarding our exposure of interest, paracetamol has a short biologic half-life, such that 24 h post-ingestion, 85–95% of the chemical has been excreted by the kidneys (Forrest et al., 1982). Therefore, the use of a single spot urine collected at study baseline to estimate preconception exposures of paracetamol is an important limitation. Nonetheless, even the use of a single urinary measurement of paracetamol may reduce potential exposure misclassification resulting from the sole reliance on questionnaire data to capture exposures, given the abundance of paracetamol in contemporary environments from a variety of sources, and given the general lack of awareness of the many medications which contain variable doses of paracetamol as a secondary ingredient (Lank, 2014). Also, we cannot ignore the potential for confounding given that information on indication of medicinal paracetamol use was not available. Ultimately, our findings need to be cautiously interpreted until corroborated by future research based on serial measurements.
Having considered the strengths and limitations of the current analysis, one question that remains unanswered is the biological mechanism explaining adult male paracetamol exposure and delayed TTP. A previous in vitro analysis of adult human testis and various analgesics reported a reduction in testosterone secretion after 24 h by 18 and 30%, in response to paracetamol exposure at 10−5 and 10−4 molar concentrations, respectively. This finding may have implications for sperm count and couple fecundity (Albert et al., 2013), but there remains a need for more research to further elucidate the mechanisms by which paracetamol affects fecundity.
Overall, we found a consistent association between higher paracetamol concentrations in men and delayed TTP, but we cannot rule out residual confounding by indication of medication use or the potential for chance findings. Our exploratory findings do, however, support the need for future studies to consider both partners' exposures when assessing couple-dependent outcomes such as TTP. Still, our findings await corroboration using research that incorporates serial collection of urinary paracetamol and detailed information on medicinal and environmental exposures in the context of couple fecundity.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
M.M.S. assisted in conceptualizing the analytic plan and paper, and wrote the first draft. K.L.G. assisted in interpretation of the data and critical revision of the paper. R.S. assisted in conceptualizing the paper, oversaw the statistical analysis, and critically discussed and revised the paper. J.M.M. implemented the statistical analysis and provided substantive edits to the paper. K.K. contributed to the acquisition of data, assisted in interpretation of the data and provided critical revision of the paper. M.H. contributed to the acquisition of data and provided substantive edits to the paper. G.M.B.L. designed the study, conceptualized the paper, assisted in interpretation of the data and provided critical revision of the paper.
Funding
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts N01-HD-3-3355, N01-HD-3-3356 and NOH-HD-3-3358). M.M.S. was supported by an Intramural Research Training Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Division of Intramural Population Health Research.
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary Material
References
- Agency for Toxic Substances and Disease Registry. Toxic Substances Portal. Atlanta, GA: US Department of Health and Human Services, Public Health Service, 2011. [Google Scholar]
- Albert O, Desdoits-Lethimonier C, Lesne L, Legrand A, Guille F, Bensalah K, Dejucq-Rainsford N, Jegou B. Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod 2013;28:1890–1898. [DOI] [PubMed] [Google Scholar]
- Bae YH, Shin JS, Lee J, Kim MR, Park KB, Cho JH, Ha IH. Association between Hypertension and the prevalence of low back pain and osteoarthritis in Koreans: a cross-sectional study. PLoS One 2015;10:e0138790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, Nieschlag E. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod 2000;15:2478–2482. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 2009;169:236–248. [DOI] [PubMed] [Google Scholar]
- Bernert JT Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, Ann Q, Covey TR, Whitfield WE, Gunter EW et al. . Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 1997;43:2281–2291. [PubMed] [Google Scholar]
- Bloom MS, Louis GMB, Sundaram R, Kostyniak PJ, Jain J. Associations between blood metals and fecundity among women residing in New York State. Reprod Toxicol 2011;31:158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde JP, Hjollund NH, Jensen TK, Ernst E, Kolstad H, Henriksen TB, Giwercman A, Skakkebaek NE, Andersson AM, Olsen J. A follow-up study of environmental and biologic determinants of fertility among 430 Danish first-pregnancy planners: design and methods. Reprod Toxicol 1998;12:19–27. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd Barr D, Schrader SM, Kim S, Chen Z et al. . Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol 2011;25:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Chen Z, Kim S, Caldwell K, Barr DB. Heavy metals and couple fecundity, the LIFE study. Chemosphere 2012;87:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Maisog J, Kim S, Chen Z, Barr DB. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect 2013;121:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir M, Samanci N, Balci N, Balci MK. Musculoskeletal manifestations in patients with thyroid disease. Clin Endocrinol (Oxf) 2003;59:162–167. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B (Methodological) 1972;34:187–220. [Google Scholar]
- Desai M, Esserman DA, Gammon MD, Terry MB. The use of complete-case and multiple imputation-based analyses in molecular epidemiology studies that assess interaction effects. Epidemiol Perspect Innov 2011;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierkes G, Weiss T, Modick H, Käfferlein HU, Brüning T, Koch HM. N-Acetyl-4-aminophenol (paracetamol), N-acetyl-2-aminophenol and acetanilide in urine samples from the general population, individuals exposed to aniline and paracetamol users. Int J Hyg Environ Health 2014;217:592–599. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, Mocarelli P. Serum dioxin concentrations and time to pregnancy. Epidemiology 2010;21:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest JA, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet 1982;7:93–107. [DOI] [PubMed] [Google Scholar]
- Gaber S. Principles of pharmacotherapy in pregnancy and lactation. In Arcangelo VP, Peterson AM (eds). Pharmacotherapeutics for Advanced Practice: A Practical Approach. Philadelphia, PA: Lippincott Williams & Wilkins, 2006, 49–54. [Google Scholar]
- Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod 2003;18:1959–1966. [DOI] [PubMed] [Google Scholar]
- Golding DN. Hypothyroidism presenting with musculoskeletal symptoms. Ann Rheum Dis 1970;29:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B. PBDE concentrations in women's serum and fecundability. Environ Health Perspect 2010;118:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005;57:79–115. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Rebordosa C, Thulstrup AM, Toft G, Sorensen HT, Bonde JP, Henriksen TB, Olsen J. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology 2010;21:779–785. [DOI] [PubMed] [Google Scholar]
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. J Am Med Assoc 2002;287:337–344. [DOI] [PubMed] [Google Scholar]
- Kerkhoff AC, Moreira LB, Fuchs FD, Fuchs SC. Association between hypertension and musculoskeletal complaints: a population-based study. J Hypertens 2012;30:2112–2117. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Hass U, Lesne L, Lottrup G, Jacobsen PR, Desdoits-Lethimonier C, Boberg J, Petersen JH, Toppari J, Jensen TK et al. . Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum Reprod 2011;26:235–244. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Lesne L, Le Fol V, Desdoits-Lethimonier C, Dejucq-Rainsford N, Leffers H, Jegou B. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid) and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl 2012;35:377–384. [DOI] [PubMed] [Google Scholar]
- Lank P. American College of Medical Toxicology. Toxicology FAQs:Acetaminophen, 2014. Retrieved from http://www.acmt.net/Acetaminophen.html (6 November 2016, date last accessed). [Google Scholar]
- Lawrence JS. Hypertension in relation to musculoskeletal disorders. Ann Rheum Dis 1975;34:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books, 1988. [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 2004;112:1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sørensen HT. Cohort profile: the Danish web-based pregnancy planning study—‘Snart-Gravid’. Int J Epidemiol 2009;38:938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modick H, Weiss T, Dierkes G, Bruning T, Koch HM. Ubiquitous presence of paracetamol in human urine: sources and implications. Reproduction 2014;147:R105–R117. [DOI] [PubMed] [Google Scholar]
- Philippat C, Giorgis-Allemand L, Chevrier C, Cordier S, Jegou B, Charles MA, Slama R. Analgesics during pregnancy and undescended testis. Epidemiology 2011;22:747–749. [DOI] [PubMed] [Google Scholar]
- Rebordosa C, Kogevinas M, Horváth-Puhó E, Nørgård B, Morales M, Czeizel AE, Vilstrup H, Sørensen HT, Olsen J. Acetaminophen use during pregnancy: effects on risk for congenital abnormalities. Am J Obstet Gynecol 2008;198:178.e171–178.e177. [DOI] [PubMed] [Google Scholar]
- Richardson DB, Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am J Epidemiol 2003;157:355–363. [DOI] [PubMed] [Google Scholar]
- Schafer J. Analysis of Incomplete Multivariate Data. New York: Chapman and Hall, 1997. [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, Liu A. The limitations due to exposure detection limits for regression models. Am J Epidemiol 2006;163:374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder CA, Kortenkamp A, Steegers EA, Jaddoe VW, Hofman A, Hass U, Burdorf A. Intrauterine exposure to mild analgesics during pregnancy and the occurrence of cryptorchidism and hypospadia in the offspring: the Generation R Study. Hum Reprod 2012;27:1191–1201. [DOI] [PubMed] [Google Scholar]
- Specht IO, Bonde JP, Toft G, Lindh CH, Jonsson BA, Jorgensen KT. Serum phthalate levels and time to pregnancy in couples from Greenland, Poland and Ukraine. PloS one 2015;10:e0120070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, Baird DD. Anti-Mullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol 2011;117:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele K, Kessler T, Arck P, Erhardt A, Tiegs G. Acetaminophen and pregnancy: short- and long-term consequences for mother and child. J Reprod Immunol 2013;97:128–139. [DOI] [PubMed] [Google Scholar]
- Tomlinson C, Marshall J, Ellis JE. Comparison of accuracy and certainty of results of six home pregnancy tests available over-the-counter. Curr Med Res Opin 2008;24:1645–1649. [DOI] [PubMed] [Google Scholar]
- Velez MP, Arbuckle TE, Fraser WD. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril 2015. a;103:1011–1020.e1012. [DOI] [PubMed] [Google Scholar]
- Velez MP, Arbuckle TE, Fraser WD. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum Reprod 2015. b;30:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA. Medication use among children <12 years of age in the United States: results from the Sloan survey. Pediatrics 2009;124:446–454. [DOI] [PubMed] [Google Scholar]
- Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol 2005;193:771–777. [DOI] [PubMed] [Google Scholar]
- White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Stat Med 2010;29:2920–2931. [DOI] [PubMed] [Google Scholar]
- Wilson J, Walton JN. Some muscular manifestations of hypothyroidism. J Neurol Neurosurg Psychiatry 1959;22:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, Gruschow SM, Horgan CE, Wiley AS, Hahn KA et al. . Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol 2015;29:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Zhang L, Chen J. Paracetamol in the environment and its degradation by microorganisms. Appl Microbiol Biotechnol 2012;96:875–884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.