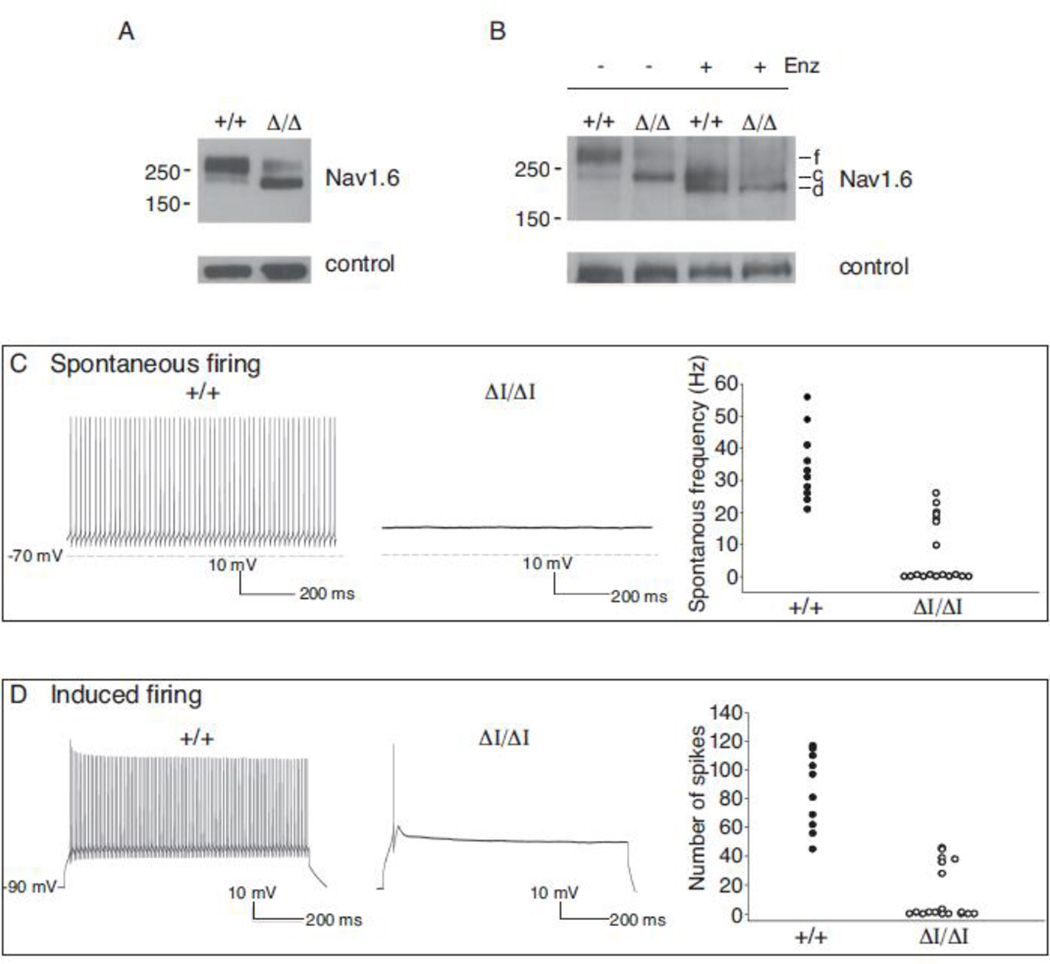
Figure 3. Partial glycosylation of Nav1.6Δ1750 and reduced channel activity.
(A) Brain membrane proteins were fractionated on acrylamide gradient gels and immunostained with polyclonal anti-Nav1.6 (Alamone #ASC-009). The apparent molecular weight of the wildtype channel corresponds to the fully glycosylated protein (f). The mutant channel migrates with lower apparent molecular weight. Digestion with peptide N-glycosidase F converted the wildtype channel to two smaller products corresponding in mobility to core-glycosylated (c) and de-glycoslyated (d) proteins. f, fully glycosylated; c, core glycosylated; d, deglycosylated. Loading controls: left, α-tubulin; right, FIG 4. (B and C) Repetitive firing of Purkinje cells in cerebellar slices is impaired in Scn8a9J/9J mutant mice. The absence of repetitive firing by mutant cells is similar to previous observations of Nav1.6 null cells (Raman et al., 1997). (D) Current density in ND7/23 cells transfected with Nav1.6-Δ1750 cDNA or wildtype Nav1.6 cDNA, alone or together with FGF13b (F).