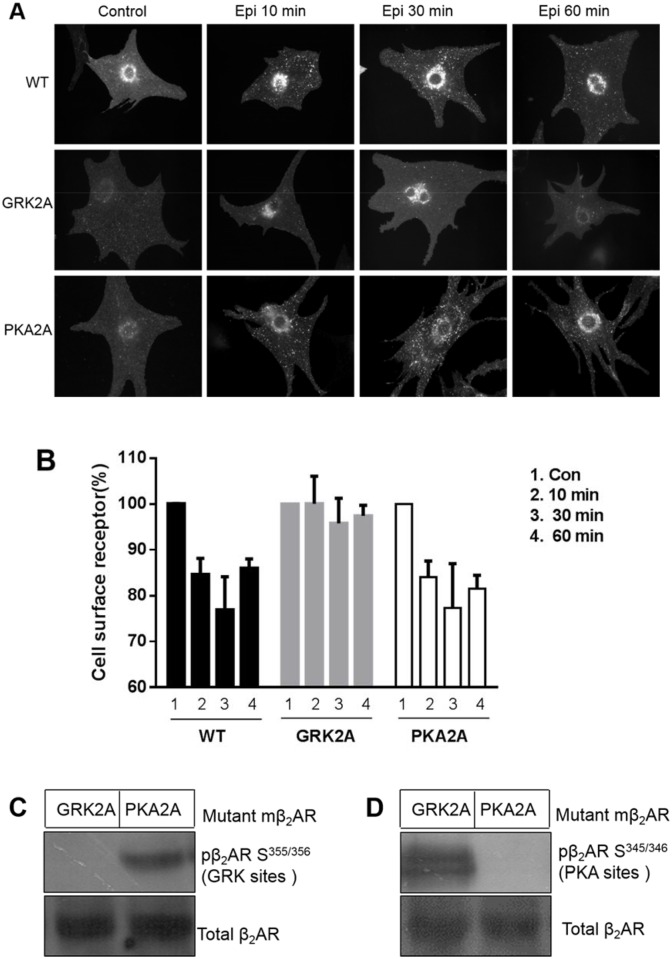
Fig 3. Mutation of β2AR S355/356 sites impaired internalization of β2ARs.
Wild-type and two mutant forms of β2AR (GRK2A and PKA2A) were expressed in neonatal cardiomyocytes isolated from β1β2AR double-knockout mice by adenovirus transfection for 2 days. (A) The cells were stimulated with Epi (10 μM) for 10, 30, or 60 min. Compared to wild-type, GRK2A β2AR (mutation at S355/356), but not PKA2A β2AR (mutation at S345/346), impaired receptor internalization. Photographs representative of 3 different preparations of cardiomyocytes are shown. (B) The cell surface receptors of wild-type or mutant β2AR were quantified by FLISA upon Epi stimulation for the indicated times. The quantitative data represent the means ± SDs of at least 3 different experiments. (C) Cardiomyocytes from β1β2AR double-knockout mice were transfected with GRK2A and PKA2A mutant β2ARs for two days and then lysed with radioimmunoprecipitation assay buffer. The lysates were subjected to SDS-PAGE followed by immunoblot analysis using a polyclonal anti-phosphoserine (355, 356)-specific or anti-phosphoserine (345, 346)-specific β2AR antibody. Phospho-β2AR Ser355/356 (GRK sites) was not observed in GRK2A (mutation of β-AR at Ser355/356), but was present in PKA2A (mutation of β-AR at Ser345/346). (D) In contrast, Phospho-β2AR Ser345/346 was not observed in PKA2A (mutation of β-AR at Ser345/346), but was present in GRK2A (mutation of β-AR at Ser355/356).