Abstract
A new sequence of pyrazole derivatives (1–6) was synthesized from condensation technique under utilizing ultrasound irradiation. Synthesized compounds were characterized from IR, 1H NMR, 13C NMR, Mass and elemental analysis. Synthesized compounds (1–6) were screened for antimicrobial activity. Among the compounds 3 (MIC: 0.25 μg/mL) was exceedingly antibacterially active against gram negative bacteria of Escherichia coli and compound 4 (MIC: 0.25 μg/mL) was highly active against gram positive bacteria of Streptococcus epidermidis compared with standard Ciprofloxacin. Compound 2 (MIC: 1 μg/mL) was highly antifungal active against Aspergillus niger proportionate to Clotrimazole. Synthesized compounds (1–6) were screened for anti-inflammatory activity and the compound 2-((5-hydroxy-3-methyl-1H-pyrazol-4-yl)(4-nitrophenyl)methyl)hydrazinecarboxamide (4) was better activity against anti-inflammatory when compared with standard drugs (Diclofenac sodium). Compounds (2, 3 and 4) are the most important molecules and hence the need to develop new drugs of antibacterial, antifungal and anti-inflammatory agents.
Keywords: Ultra sound irradiation, Pyrazole derivatives, Mannich bases, Antimicrobial activity, Anti-inflammatory activity, Structure–activity Relationships (SAR)
1. Introduction
The pyrazole moiety is a versatile lead molecule in the pharmaceutical development and has a wide range of biological activities (Goda et al., 2003; El-Emary, 2006; Mansour et al., 2003), antibacterial (Sangapure et al., 2001), antifungal (Gupta et al., 2005, Ashish et al., 2006) and pharmacological activities such as anti-inflammatory (Makhsumov et al., 1986), antitubercular (Chetan and Mulwar, 2000), anticancer (Nimavat and Popat, 2007), analgesic (Udupi et al., 1998), antipyretic (Fabiane et al., 2002), anticonvulsant (Ashok et al., 2001) activities.
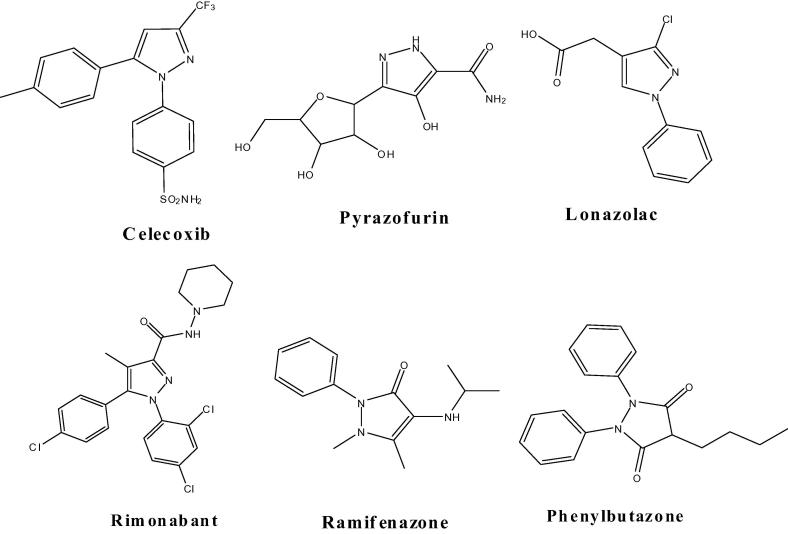
Commercially available pyrazole moiety (Fig. 1) such as Celecoxib is potent COX-2 inhibitor (Penning et al., 1997). Some other examples of pyrazole derivatives as NSAID are ramifenazone (Fioravanti et al., 2010), Lonazolac (NSAID) (Riedel, 1981) and Rimonabant (Isidro and Cordido, 2009). Compound (phenylbutazone) is a non steroidal drug (Reed et al., 1985, Vennerstorm et al., 1987). Pyrazofurin is potential of antiviral activity, HCV virus (Rostom et al., 2003, Riyadh et al., 2010, Popovici-Muller et al., 2009, Farghaly et al., 2011). In the current research, anti-inflammatory drug has been used in most prominent research areas. New anti-inflammatory drugs were previously used in clinical research, some of the drugs are still not efficient and have intolerable side effects.
Figure 1.
The structures of some drugs bearing the pyrazole moiety.
Based on the above study, we need to develop new drugs against anti-inflammatory and antimicrobial activities. Therefore, we were led to identify new approaches of pyrazole derivatives as well as test the antimicrobial and anti-inflammatory activity.
2. Methods and materials
2.1. Chemicals and reagents
All chemicals were acquired from Sigma–Aldrich Chemical Co (Sigma–Aldrich Corp., St. Louis, MO, USA). The Infrared spectra (KBr), Proton NMR, Carbon NMR, Mass spectra (EI), and Elemental analysis (C, H, N and S) were recorded using Shimadzu 8201PC (4000–400 cm−1), Bruker DRX-400 MHz, Jeol JMS D-300 spectrometer operating, and Elementer analyser model (Varian EL III).
2.1.1. Synthesis of 2-((5-hydroxy-3-methyl-1H-pyrazol-4-yl)(phenyl)methyl)hydrazinecarboxamide (1)
A mixture of 5-hydroxy-3-methyl-1H-pyrazoles (0.1 mol), benzaldehyde (0.1 mol) and semicarbazide hydrochloride (0.1 mol) was treated with ultrasound irradiation under ethanol medium. After completion of reaction, the product was isolated and identified by TLC. The identified product was separated from column chromatography and recrystallized by suitable solvent. The above experiential procedure was pursued by remaining compounds 2–6.
IR (cm−1): 3445 (C–OH), 670(–CH–), 1679 (NH CO), 1569 (NH2). 1H NMR (DMSO-d6): δ 9.90 (s, 1H, C–OH), 2.71 (d, J = 4.4 Hz, 1H, CH), 2.23(s, 3H,CH3), 4.45 (dd, J = 5.3 Hz, 1H, –CH–), 7.33–7.49 (m, 5H, Phenyl ring), 2.36 (d, J = 2.0 Hz, 1H, NH), 6.81 (d, J = 1.4 Hz, 1H, NH), 6.25 (s, 2H, NH2). 13C NMR (CDCl3): δ 167.2(C–OH), 162.6 (C–CH3), 42.2(C–CH–), 52.3(C–CH–NH), 18.7(C–CH3), 155.4(CONH2), 141.7, 112.0, 129.2, 133.8 (Phenyl ring). Mass (m/z): 261.27 (M+, 32%), 244.28, 216.38, 200.27 (100%), 185.26, 170.25, 94.15.
2.1.2. 2-[(4-chlorophenyl)(3-hydroxy-5-methyl-4H-pyrazol-4-yl)methyl]hydrazinecarboxamide(2)
IR (cm−1): 3469 (C–OH), 691(–CH–), 1665 (NH CO), 1554(NH2), 897(C–Cl). 1H NMR (DMSO-d6): δ 9.96(s, 1H, C–OH), 2.76 (d, J = 4.3 Hz, 1H, CH), 2.29 (s, 3H, CH3), 4.30(dd, J = 5.2 Hz, 1H, –CH–), 7.21–7.39 (dd, 4H, Phenyl ring), 2.31 (s, J = 2.1 Hz, 1H, NH), 6.73 (s, J = 1.6 Hz, 1H, NH), 6.19 (s, 2H, NH2). 13C NMR (CDCl3): δ 167.2 (C–OH), 162.6 (C–CH3), 42.2 (C–CH–), 52.3 (C–CH–NH), 18.7 (C–CH3), 155.4 (CONH2), 141.7, 112.0, 129.2, 133.8 (Phenyl ring). Mass (m/z): 295.72(M+, 26%), 277.74, 243.30 (100%), 228.28, 200.27, 185.26, 170.25, 94.15.
2.1.3. 2-((3-hydroxy-5-methyl-4H-pyrazol-4-yl)(4-hydroxyphenyl)methyl)hydrazinecarboxamide (3)
IR(cm−1): 3457(C–OH), 682(–CH–), 1661(NHCO), 1508(NH2), 1447(OH).1H NMR(DMSO-d6): δ 9.89 (s, 1H, C–OH), 2.70(d, J = 4.1 Hz, 1H,CH), 2.31 (s, 3H, CH3), 4.36(dd, J = 5.6 Hz, 1H, –CH–), 7.23–7.31 (dd, 4H, Phenyl ring), 2.33 (s, J = 2.3 Hz, 1H, NH), 6.70 (s, J = 1.6 Hz, 1H, NH), 6.21 (s, 2H, NH2). 13C NMR (CDCl3): δ 167.9 (COH), 163.2 (C2–CH3), 42.6 (C–CH–), 51.9 (C–CH–NH), 18.5 (C–CH3), 155.0 (CONH2), 146.7, 111.5, 128.2, 137.2 (Phenyl ring). Mass (m/z): 277.27(M+, 22%), 259.30, 243.42(100%), 228.28, 200.27, 185.26, 170.25.
2.1.4. 2-[(3-hydroxy-5-methyl-4H-pyrazol-4-yl)(4-nitrophenyl)methyl]hydrazinecarboxamide (4)
IR(cm−1); 3450 (C–OH), 679(–CH–), 1660 (NHCO), 1514(NH2), 1536(C–NO2). 1H NMR (DMSO-d6): δ9.91 (s, 1H, C–OH), 2.79(d, J = 4.6 Hz, 1H, CH), 2.32 (s, 3H, CH3), 4.41 (dd, J = 5.2 Hz, 1H, –CH–), 7.29–7.42 (dd, 4H, Phenyl ring), 2.39 (d, J = 2.1 Hz, 1H, NH), 6.81 (d, J = 1.7 Hz, 1H, NH), 6.26 (s, 2H, NH2). 13C NMR (CDCl3): δ167.1(C–OH), 162.9(C–CH3), 42.0 (C–CH–), 52.6 (C–CH–NH), 19.8 (C–CH3), 156.3 (CONH2), 142.9, 112.5, 128.2, 131.2 (Phenyl ring). Mass (m/z): 306.27(M+, 26%), 289.28, 261.27, 245.27(100%), 200.27, 185.26, 170.25.
2.1.5. 2-[(3-hydroxy-5-methyl-4H-pyrazol-4-yl)(4-methoxy phenyl)methyl]hydrazine carboxamide(5)
IR (cm−1): 3460 (C–OH), 682(–CH–), 1668 (NHCO),1508 (NH2).1H NMR (DMSO-d6): δ 9.92 (s, 1H, C–OH), 2.76 (d, J = 4.1 Hz, 1H, CH),2.36 (s, 3H, CH3),4.28 (dd, J = 5.5 Hz, 1H, –CH–),7.19–7.25 (dd, 4H, Phenyl ring), 2.39 (d, J = 2.2 Hz, 1H, NH), 6.84(d, J = 1.5 Hz,1H, NH), 6.15(s,2H, NH2).13C NMR (CDCl3): δ 167.3 (C–OH), 162.0 (C–CH3), 42.9 (C–CH–), 53.1 (C–CH–NH), 19.1 (C–CH3), 156.8 (CONH2),141.7,112.0,129.2, 133.8 (Phenyl ring). Mass (1 m/z): 291.30(M+, 41%), 259.30, 243.30, 200.27(100%), 185.26, 170.25, 94.15.
2.1.6. 2-((4-(dimethylamino)phenyl)(3-hydroxy-5-methyl-4H-pyrazol-4-yl)methyl)hydrazinecarboxamide (6)
IR (cm−1): 3451 (C–OH), 663(–CH–), 1679 (NHCO), 1512 (NH2). 1H NMR (DMSO-d6): δ 9.95 (s, 1H, C–OH), 2.76 (d, J = 4.3 Hz, 1H, CH), 2.24 (s, 3H, CH3), 4.41(dd, J = 5.6 Hz, 1H, –CH–),7.29–7.41 (dd, 4H, Phenyl ring), 2.36 (d, J = 2.3 Hz, 1H, NH), 6.82(d, J = 1.9 Hz, 1H, NH), 6.17 (s, 2H, NH2). 13C NMR (CDCl3): δ167.9 (C–OH), 162.5(C–CH3), 43.1(C–CH–), 54.6 (C–CH–NH), 18.9(C–CH3), 157.1 (CONH2), 142.8, 113.0, 127.2, 134.1 (Phenyl ring). Mass (m/z): 304.34 (M+, 27%), 286.37, 276.30, 243.34(100%), 200.27, 185.26, 170.25, 94.15.
2.2. Pharmacological activity
2.2.1. Anti-inflammatory activity
Isolated compounds (1–6) were evaluated by anti-inflammatory activity, screening method followed from the literature method (Winter et al., 1962). Albino rats of both sexes weighing 150 g were divided into 4 groups, each group consists of 5 animals. Inflammation was induced by intra planter injection of histamine (0.1 mL of 1% histamine for induction of paw edema). The rats are challenged by s.c injection of 0.1 mL of 1% solution of histamine into the sub-plantar side of the left hind paw. 1 h after the administration of the test compounds (10 mg/kg; p.o), one group was kept as control, received only 0.5% carboxy methyl cellulose solution. The volume was measured before and after 3 h of carrageen treatment by means of plethysmometer.
The percentage of anti-inflammatory activity was calculated by = (Vc − Vt/Vc) × 100.Vc = control, Vt = test sample.
2.2.2. In vitro antibacterial screening
The antibacterial screening for isolated compounds was determined by the disc diffusion method (Bauer et al., 1996) using Mueller–Hinton agar (Hi-Media) medium. The synthesized compounds were evaluated against gram negative bacteria of Escherichia coli (MTCC-739), Pseudomonas aeruginosa (MTCC-2435), Klebsiella pneumonia (recultured) and gram positive bacteria of Streptococcus epidermidis, Staphylococcus aureus (MTCC-96). Synthesized compounds were initially screened by maximum concentration at 100 μg/mL in DMSO. The zone of inhibition was measured after 24 h incubation at 37 °C. The MIC was identified by twofold dilutions of the solution method (64, 32…., 0.5 μg/mL).
2.2.3. In vitro antifungal screening
The antifungal screening for isolated compounds was determined by using the disc diffusion method (Verma et al., 1998) with Sabouraud’s dextrose agar (Hi-Media). The isolated compounds were estimated for their in vitro antifungal activity against Aspergillus niger, Candia albicans, Microsporum audouinii and Cryptococcus neoformans (recultured). Synthesized compounds were initially screened by maximum concentration at 100 μg/mL in DMSO. The zone of inhibition (mm) was measured after incubation at 37 °C. The MIC was identified by twofold dilutions of the solution method (64, 32…., 0.5 μg/mL).
3. Results
3.1. Synthesis and characterization of pyrazole analogues
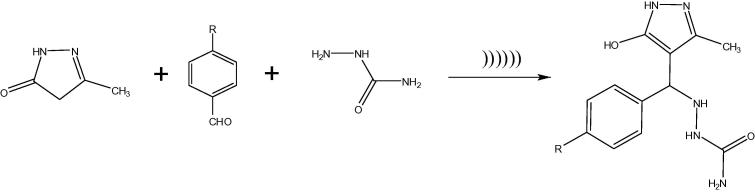
Title compounds (1–6) were synthesized from 5(3)-hydroxy-3(5)-methyl-1H-pyrazoles reacting with aldehyde and semicarbazide via Ultrasound irradiation under aqueous medium and without catalysis condition, the synthetic route of pyrazole derivatives is represented in Scheme 1. The compounds (1–6) were manufactured by Mannich base condensation method and the mechanism of the work outlined in Scheme 1. Physicochemical data of the compounds (1–6) are given in Table 1.
Scheme 1.
Synthetic route of the isolated compounds (1–6).
Table 1.
Physicochemical data of the compounds (1–6).
| Comp. No. | Ar | Yield % | mp°C | m.f | m.w | Elemental analysis calculated (found) |
||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| 1 | –H | 87 | 161 | C12H15N5O2 | 261.27 | 55.16 (55.20) | 5.79 (5.71) | 26.80 (26.79) |
| 2 | –Cl | 78 | 89 | C12H14ClN5O2 | 295.72 | 44.74 (44.72) | 4.77 (4.76) | 23.68 (23.65) |
| 3 | –OH | 81 | 121 | C12H15N5O3 | 277.27 | 51.98 (51.97) | 5.45 (5.40) | 25.26 (25.23) |
| 4 | –NO2 | 72 | 134 | C12H14N6O4 | 306.27 | 47.06 (47.10) | 4.61 (4.59) | 27.44 (27.43) |
| 5 | –OCH3 | 81 | 110 | C13H17N5O3 | 291.30 | 53.60 (53.65) | 5.88 (5.86) | 24.04 (24.08) |
| 6 | –N(CH3)2 | 85 | 97 | C14H20N6O2 | 304.34 | 55.25 (55.30) | 6.62 (6.60) | 27.61 (27.60) |
Isolated compounds were characterized by Infra red, Proton NMR, Carbon NMR spectrum, Mass spectra, and elemental analyses.
Compound 1 was confirmed by IR spectral analysis, which indicates the value of 3445 cm−1 corresponding to the OH group nearby in the pyrazole ring and another absorption band at 670 cm−1 corresponding to the –CH– group presented in the pyrazole ring. Another analysis of 1H NMR spectrum shows the signals observed at δ 13.03, 11.43, 5.45, 2.36, and 6.25 corresponding to NH in the pyrazole ring, C–OH, –CH–, NH, CH, and NH2 protons respectively and 13C NMR spectrum shows that peaks at δ 132.2, 51.2, and 12.7 correspond to C–OH, C–CH–NH, and CH3 carbons respectively. Molecular mass of compound 1 was confirmed by mass spectral analysis, which is indicated that the molecular ion peaks at 261.27(M+, 32%).
3.2. Anti-inflammatory activity
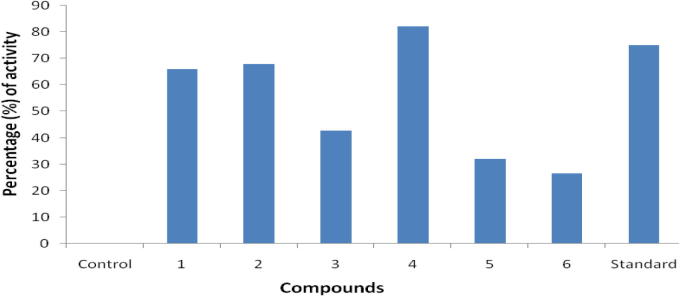
Isolated products (1–6) were evaluated for anti-inflammatory activity match up with diclofenac sodium at oral dose. Rat albino was used as an oral dose of the test compound at a concentration of 10 mg/kg, the percentage of the activity was measured at 3 h. Compound (4) is highly active compared with standard. Fig. 2 shows the activity difference of compounds (1–6), anti-inflammatory activity records are presented in Table 2.
Figure 2.
Anti-inflammatory activity of compounds (1–6).
Table 2.
Anti-inflammatory activity of compounds (1–6).
| Comp. No | Increase in paw volume (3hr – 0hr) | Percentage (%) of Activity, Dose (10 mg/ kg) |
|---|---|---|
| Control | 0.56 | – |
| 1 | 0.22 ± 0.05 | 66.0∗ |
| 2 | 0.18 ± 0.06 | 67.8∗ |
| 3 | 0.32 ± 0.06 | 42.8∗ |
| 4 | 0.10 ± 0.02 | 82.1∗ |
| 5 | 0.38 ± 0.09 | 32.1∗ |
| 6 | 0.41 ± 0.02 | 26.7∗ |
| Standard | 0.14 ± 0.01 | 75.0∗ |
Mean ± SEM, n = 6 in each group. Significance levels ∗P < 0.01 as compared with the respective control. Diclofenac sodium was used as a standard.
3.3. Antibacterial activity
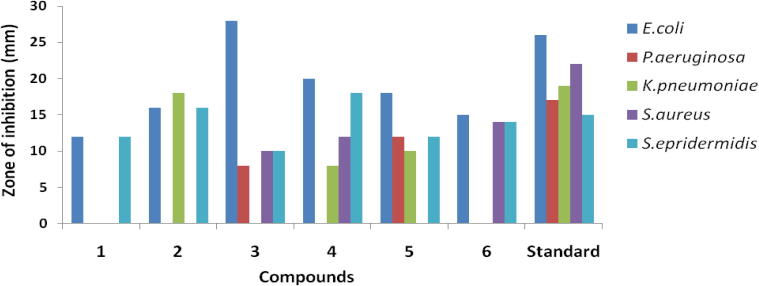
Compound (3) is exceedingly active (MIC: 0.25 μg/mL) against gram negative bacteria of E. coli compared with Ciprofloxacin MIC: 0.5 μg/mL. Compound (4) (MIC: 0.25 μg/mL) is highly active and match up with standard (MIC: 4 μg/mL) against gram-negative bacteria of S. epidermidis. The bacterial zones of inhibition values are presented in Table 3 and Fig. 3 indicates differentiation of antibacterial activity in isolated compounds (1–6).
Table 3.
Antibacterial activity of isolated products (1–6).
| Compounds | Gram-negative |
Gram-positive |
|||
|---|---|---|---|---|---|
| E. coli | P. aeruginosa | K. pneumoniae | S. aureus | S. epidermidis | |
| 1 | 12 | – | – | – | 12 |
| 2 | 16 | – | 18 | – | 16 |
| 3 | 28 | 8 | – | 10 | 10 |
| 4 | 20 | – | 8 | 12 | 18 |
| 5 | 18 | 12 | 10 | - | 12 |
| 6 | 15 | – | – | 14 | 14 |
| Standard | 26 | 17 | 19 | 22 | 15 |
Ciprofloxacin used as a standard, zone of inhibition measured at (mm).
Figure 3.
Antibacterial activity of compounds (1–6).
3.4. Antifungal activity
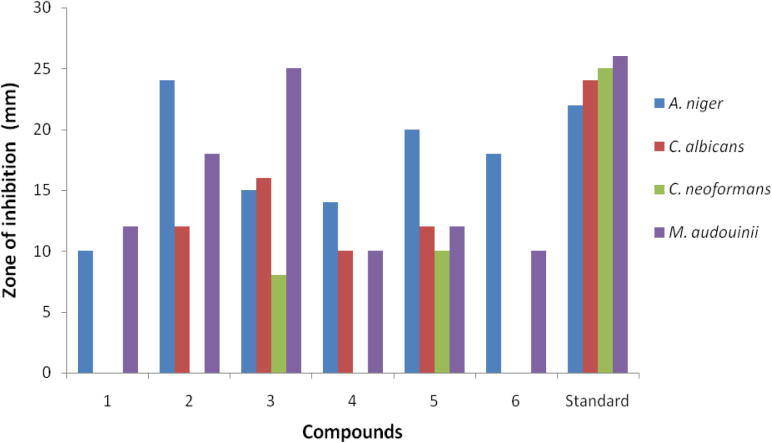
Compound (2) MIC: 1 μg/mL is greatly active against A. niger match up to with Clotrimazole MIC: 2 μg/mL. Compound (3) (MIC: 0.5 μg/mL) has equipotent activity against M. audouinii match up to with standard Clotrimazole (MIC: 0.5 μg/mL) whereas compound 5 (MIC: 4 μg/mL) is moderately active against A. niger. The fungal zones of inhibition values are presented in Table 4. Fig. 4 indicates differentiation of antifungal activity in isolated compounds of compounds (1–6). Minimal inhibitory concentration (MIC) data are reported in Table 5.
Table 4.
Antifungal activity of isolated products (1–6).
| Compounds | A. niger | C. albicans | C. neoformans | M. audouinii |
|---|---|---|---|---|
| 1 | 10 | – | – | 12 |
| 2 | 24 | 12 | – | 18 |
| 3 | 15 | 16 | 8 | 25 |
| 4 | 14 | 10 | – | 10 |
| 5 | 20 | 12 | 10 | 12 |
| 6 | 18 | – | – | 10 |
| Standard | 22 | 24 | 25 | 26 |
Clotrimazole used as a standard, zone of inhibition measured at (mm).
Figure 4.
Antifungal activity of compounds (1–6).
Table 5.
Minimum inhibition concentration of isolated products (2, 3 and 5).
| Comp. No. | E. c | P. a | K. p | S. a | S. e | A. n | C. a | C. n | M. a |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 64 | >100 | 1 | >100 | 16 | 1 | 64 | >100 | 16 |
| 3 | 0.25 | 64 | >100 | 64 | 64 | 64 | 64 | >100 | 0.5 |
| 4 | 16 | >100 | >100 | 64 | 2 | 64 | >100 | >100 | >100 |
| 5 | 16 | 32 | 64 | >100 | 16 | 4 | 64 | 64 | 32 |
| Ciprofloxacin | 0.5 | 1 | 2 | 0.5 | 4 | – | – | – | – |
| Clotrimazole | – | – | – | – | – | 2 | 1 | 0.5 | 0.5 |
4. Discussion
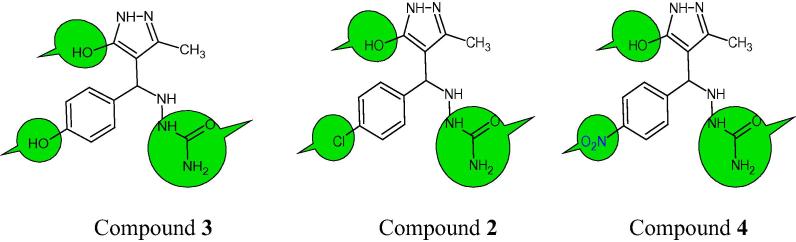
The anti-inflammatory and antimicrobial performances of the isolated compounds were confirmed by structure–activity relationships (Fig. 5).
Figure 5.
Structure–activity relationships for compounds (3, 2 and 4).
The 4-substituted phenyl ring with semicarbazone acts as a lipophilic and hydrogen bonding domain. Therefore, the above group containing the pyrazole ring may be stated as the essential pharmacophoric requirement for anti-inflammatory activity.
(i) Compound 3 (MIC: 0.25 μg/mL) pyrazole derivative exhibited high activity against gram negative E. coli compared with standard Ciprofloxacin and also antifungal activity of compound 3 exhibited equipotent activity (MIC: 0.5 μg/mL) against M. audouinii match up to with standard Clotrimazole (MIC: 0.5 μg/mL), reason of activity due to the presence of 4-OH-phenyl with semicarbazone and pyrazole moiety in compound 3. (ii) Compound 2 (MIC: 1 μg/mL) is highly active against gram negative bacteria of K. pneumoniae and also highly active (MIC: 1 μg/mL) against A. niger compared with standard Clotrimazole (MIC: 2 μg/mL) in antifungal screening, 4-Cl-phenyl with semicarbazone and pyrazole moiety were represented to be highly active against K. pneumoniae bacterial and A. niger fungal strain. (iii) Compound 4 is highly active (MIC: 2 μg/mL) against gram positive bacteria of S. epidermidis compared with standard Ciprofloxacin (MIC: 4 μg/mL), reason of activity due to the presence of 4-NO2-phenyl with semicarbazone and pyrazole moiety in compound 4. The compound 4 is very much active (82%) against anti-inflammatory in relation to Diclofenac sodium 74.0% of activity.
5. Conclusion
In conclusion, synthesized compounds (1–6) were tested with anti-inflammatory and antimicrobial screening. Among the series, compound (3) (MIC : 0.25 μg/mL) was found to be the most active against gram negative bacterial strain E. coli compared with standard Ciprofloxacin, antifungal activity of compound (2) (MIC: 1 μg/mL) was greatly active against A. niger than standard Clotrimazole and compound (4) showed better activity against anti-inflammatory when compared with Diclofenac sodium. Therefore these derivatives could provide as a highly momentous molecule for further development of antimicrobial, anti-inflammatory agents.
Acknowledgment
We are very grateful to Saudi Biological Society and Prince Sultan Research Chair for Environment and Wildlife, Department of Botany & Microbiology, College of Sciences, King Saud University (KSU), Riyadh, Saudi Arabia for encouragement and support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ashish K.T., Anil M., Verma H.N., Mishra A. Synthesis and antifungal activity of 4-substituted-3,7-dimethyl pyrazolo[3,4-e] [1,2,4] triazine. Ind. J. Chem. 2006;45B:489–492. [Google Scholar]
- Ashok K., Archana, Sharma S. Synthesis of potential quinazolinyl pyrazolines as anticonvulsant agents. Ind. J. Hetero. Chem. 2001;9:197. [Google Scholar]
- Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1996;39(5):493–496. [PubMed] [Google Scholar]
- Chetan B.P., Mulwar V.V. Synthesis and evaluation of certain pyrazolines and related compounds for their anti tubercular, anti bacterial and anti fungal activities. Ind. J. Chem. 2000;44B:232–237. [Google Scholar]
- El-Emary T.I. Synthesis and biological activity of some new pyrazole[3,4-b]pyrazines. J. Chin. Chem. Soc. 2006;53:391–401. [Google Scholar]
- Fabiane R.S., Vanessa T.S., Viviane R., Lysandro P.B., Marli R.O., Helio G.B., Nilo Z., Marcos A.P.M., Carlos F.M. Hypothermic and antipyretic effects of 3-methyl- and 3-phenyl-5-hydroxy-5- trichloromethyl-4,5-dihydro-1H-pyrazole-1-carboxyamides in mice. Eur. J. Pharmacol. 2002;451:141–147. doi: 10.1016/s0014-2999(02)02225-2. [DOI] [PubMed] [Google Scholar]
- Farghaly T.A., Abass I.M., Abdalla M.M., Mahgoub R.O.A. Synthesis and pharmacological activities of fused pyrimidinones. World J. Chem. 2011;6:8–18. [Google Scholar]
- Fioravanti R., Bolasco A., Manna F., Rossi F., Orallo F., Ortuso F., Alcaro S., Cirilli R. Synthesis and biological evaluation of N-substituted-3,5-diphenyl-2-pyrazoline derivatives as cyclooxygenase (COX-2) inhibitors. Eur. J. Med. Chem. 2010;45:6135–6138. doi: 10.1016/j.ejmech.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Goda F.E., Maarouf A.R., El-Bendary E.R. Synthesis and antimicrobial evaluation of new isoxazole and pyrazole. Saudi Pharm. J. 2003;11:111–117. [Google Scholar]
- Gupta U., Sareen V., Khatri V., Chug S. Synthesis and antifungal activity of new fluorine containing 4-pyrazole and isoxazole. Ind. J. Heterocycl. Chem. 2005;14:265–266. [Google Scholar]
- Isidro M.L., Cordido F. Drug treatment of obesity: established and emerging therapies. Mini Rev. Med. Chem. 2009;9:664–673. doi: 10.2174/138955709788452739. [DOI] [PubMed] [Google Scholar]
- Makhsumov A.D., Dzhurae Kilichov G., Nikbae A.T. Anti-inflammatory Activity of some pyrazole derivatives. Pharm. Chem. J. 1986;20:289–291. [Google Scholar]
- Mansour A.K., Eid M.M., Khalil S.A.M.N. Synthesis and reactions of some new heterocyclic carbohydrazides and related compounds as potential anticancer agents. Molecules. 2003;8:744–755. [Google Scholar]
- Nimavat K.S., Popat K.H. Synthesis anticancer, antitubercular and antimicrobial activities of 1- substituted, 3-aryl-5-(3′-bromophenyl) pyrazoline. Ind. J. Heterocycl. Chem. 2007;16:333–336. [Google Scholar]
- Penning T.D., Penning T.D., Talley J.J., Bertenshaw S.R., Carter J.S., Collins P.W., Docter S., Graneto M.J., Lee L.F., Malecha J.W., Miyashiro J.M., Rogers R.S., Rogier D.J., Yu S.S., Anderson G.D., Burton E.G., Cogburn J.N., Gregory S.A., Koboldt C.M., Perkins W.E., Seibert K., Veenhuizen A.W., Zhang Y.Y., Isakson P.C. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, celecoxib) J. Med. Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- Popovici-Muller J., Shipps G.W., Jr., Rosner K.E., Deng Y., Wang T., Curran P.J., Brown M.A., Siddiqui M.A., Cooper A.B., Duca J. Pyrazolo[1,5-a]pyrimidine-based inhibitors of HCV polymerase. Bioorg. Med. Chem. 2009;19:6331–6336. doi: 10.1016/j.bmcl.2009.09.087. [DOI] [PubMed] [Google Scholar]
- Reed G.A., Griffin I.O., Eling T.E. Inactivation of prostaglandin H synthase and prostacyclin syntheses by phenylbutazone. Requirement for peroxidative metabolism. Mol. Pharmacol. 1985;27:109–114. [PubMed] [Google Scholar]
- Riedel R. Lonazolac-Ca = Calcium [3-(p-chlorophenyl)-1-phenylpyrazole-4[-acetate 1 Pharmacological properties of a new antiinflammatory/antirheumatic drug (author’s transl) Arzneimittelforschung. 1981;31:655–665. [PubMed] [Google Scholar]
- Riyadh S.M., Farghaly T.A., Abdallah M.A., Abdalla M.M., Abd El-Aziz M.R. New pyrazoles incorporating pyrazolylpyrazole moiety Synthesis, anti-HCV and antitumor activity. Eur. J. Med. Chem. 2010;45:1042–1050. doi: 10.1016/j.ejmech.2009.11.050. [DOI] [PubMed] [Google Scholar]
- Rostom S.A., Shalaby M.A., El-Demellawy M.A. Polysubstituted pyrazoles, part 5. Synthesis of new 1-(4-chlorop enyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide analogs and some derived ring systems. A novel class of potential antitumor and anti-HCV agents. Eur. J. Med. Chem. 2003;38:959–974. doi: 10.1016/j.ejmech.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Sangapure S.S., Bodke Y., Raga B. Synthesis of some new pyrazolines as potential antimicrobial agents. Ind. J. Heterocycl. Chem. 2001;11:31–37. [Google Scholar]
- Udupi R.H., Bhat A.R., Krishna K. Synthesis and investigation of some new pyrazoline derivatives for their antimicrobial, anti inflammatory and analgesic activities. Ind. J. Heterocycl. Chem. 1998;8:143–146. [Google Scholar]
- Vennerstorm J.L., Holmes T.J., Jr. Preparation and evaluation of electrophilic derivatives of phenylbutazone as inhibitors of prostaglandin-H-synthase. J. Med. Chem. 1987;30:563–567. doi: 10.1021/jm00386a020. [DOI] [PubMed] [Google Scholar]
- Verma, R.S., Khan, I.K., Singh, A.P., 1998. Antifungal agents, past, present, future prospects, National Academy of Chemistry and Biology, Lucknow, India. pp. 55–128.
- Winter C.A., Risley E.A., Nuss G.N. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]